Combined aclidinium bromide and long-acting beta2-agonist for chronic obstructive pulmonary disease (COPD)
- PMID: 30536566
- PMCID: PMC6517126
- DOI: 10.1002/14651858.CD011594.pub2
Combined aclidinium bromide and long-acting beta2-agonist for chronic obstructive pulmonary disease (COPD)
Abstract
Background: Several dual bronchodilator combinations of long-acting beta2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) have been approved for treatment of stable chronic obstructive pulmonary disease (COPD). The current GOLD (Global Initiative for Chronic Obstructive Lung Disease) recommendations suggest the use of LABA/LAMA combinations in people with group B COPD with persistent symptoms, group C COPD with further exacerbations on LAMA therapy alone and group D COPD with or without inhaled corticosteroids (ICS). Fixed-dose combination (FDC) of aclidinium/formoterol is one of the approved LABA/LAMA therapies for people with stable COPD.
Objectives: To assess the efficacy and safety of combined aclidinium bromide and long-acting beta2-agonists in stable COPD.
Search methods: We searched the Cochrane Airways Group Specialised Register (CAGR), ClinicalTrials.gov, World Health Organization (WHO) trials portal, United States Food and Drug Administration (FDA) and manufacturers' websites as well as the reference list of published trials up to 12 October 2018.
Selection criteria: Parallel-group randomised controlled trials (RCTs) assessing combined aclidinium bromide and LABAs in people with stable COPD.
Data collection and analysis: We used standard methodological procedures expected by Cochrane for data collection and analysis. The primary outcomes were exacerbations requiring a short course of an oral steroid or antibiotic, or both; quality of life measured by a validated scale and non-fatal serious adverse events (SAEs). Where the outcome or study details were not reported, we contacted the study investigators or pharmaceutical company trial co-ordinators (or both) for missing data.
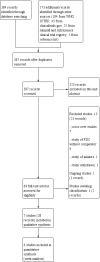
Main results: We identified RCTs comparing aclidinium/formoterol FDC versus aclidinium, formoterol or placebo only. We included seven multicentre trials of four to 52 weeks' duration conducted in outpatient settings. There were 5921 participants, whose mean age ranged from 60.7 to 64.7 years, mostly men with a mean smoking pack-years of 46.4 to 61.3 of which 43.9% to 63.4% were current smokers. They had a moderate-to-severe degree of COPD with a mean postbronchodilator forced expiratory volume in one second (FEV1) between 50.5% and 61% of predicted normal and the baseline mean FEV1 of 1.23 L to 1.43 L. We assessed performance and detection biases as low for all studies whereas selection, attrition and reporting biases were either low or unclear.FDC versus aclidiniumThere was no evidence of a difference between FDC and aclidinium for exacerbations requiring steroids or antibiotics, or both (OR 0.95, 95% CI 0.71 to 1.27; 2 trials, 2156 participants; moderate-certainty evidence); quality of life measured by St George's Respiratory Questionnaire (SGRQ) total score (MD -0.92, 95% CI -2.15 to 0.30); participants with significant improvement in SGRQ score (OR 1.17, 95% CI 0.97 to 1.41; 2 trials, 2002 participants; moderate-certainty evidence); non-fatal SAE (OR 1.19, 95% CI 0.79 to 1.80; 3 trials, 2473 participants; moderate-certainty evidence); hospital admissions due to severe exacerbations (OR 0.62, 95% CI 0.29 to 1.29; 2 trials, 2156 participants; moderate-certainty evidence) or adverse events (OR 0.95, 95% CI 0.76 to 1.18; 3 trials, 2473 participants; moderate-certainty evidence). Compared with aclidinium, FDC improved symptoms (Transitional Dyspnoea Index (TDI) focal score: MD 0.37, 95% CI 0.07 to 0.68; 2 trials, 2013 participants) with a higher chance of achieving a minimal clinically important difference (MCID) of at least one unit improvement (OR 1.34, 95% CI 1.11 to 1.62; high-certainty evidence); the number needed to treat for an additional beneficial outcome (NNTB) being 14 (95% CI 9 to 39).FDC versus formoterolWhen compared to formoterol, combination therapy reduced exacerbations requiring steroids or antibiotics, or both (OR 0.78, 95% CI 0.62 to 0.99; 3 trials, 2694 participants; high-certainty evidence); may decrease SGRQ total score (MD -1.88, 95% CI -3.10 to -0.65; 2 trials, 2002 participants; low-certainty evidence; MCID for SGRQ is 4 units); increased TDI focal score (MD 0.42, 95% CI 0.11 to 0.72; 2 trials, 2010 participants) with more participants attaining an MCID (OR 1.30, 95% CI 1.07 to 1.56; high-certainty evidence) and an NNTB of 16 (95% CI 10 to 60). FDC lowered the risk of adverse events compared to formoterol (OR 0.78, 95% CI 0.65 to 0.93; 5 trials, 3140 participants; high-certainty evidence; NNTB 22). However, there was no difference between FDC and formoterol for hospital admissions, all-cause mortality and non-fatal SAEs.FDC versus placeboCompared with placebo, FDC demonstrated no evidence of a difference in exacerbations requiring steroids or antibiotics, or both (OR 0.82, 95% CI 0.60 to 1.12; 2 trials, 1960 participants; moderate-certainty evidence) or hospital admissions due to severe exacerbations (OR 0.55, 95% CI 0.25 to 1.18; 2 trials, 1960 participants; moderate-certainty evidence), although estimates were uncertain. Quality of life measure by SGRQ total score was significantly better with FDC compared to placebo (MD -2.91, 95% CI -4.33 to -1.50; 2 trials, 1823 participants) resulting in a corresponding increase in SGRQ responders who achieved at least four units decrease in SGRQ total score (OR 1.72, 95% CI 1.39 to 2.13; high-certainty evidence) with an NNTB of 7 (95% CI 5 to 12). FDC also improved symptoms measured by TDI focal score (MD 1.32, 95% CI 0.96 to 1.69; 2 studies, 1832 participants) with more participants attaining at least one unit improvement in TDI focal score (OR 2.51, 95% CI 2.02 to 3.11; high-certainty evidence; NNTB 4). There were no differences in non-fatal SAEs, adverse events and all-cause mortality between FDC and placebo.Combination therapy significantly improved trough FEV1 compared to aclidinium, formoterol or placebo.
Authors' conclusions: FDC improved dyspnoea and lung function compared to aclidinium, formoterol or placebo, and this translated into an increase in the number of responders on combination treatment. Quality of life was better with combination compared to formoterol or placebo. There was no evidence of a difference between FDC and monotherapy or placebo for exacerbations, hospital admissions, mortality, non-fatal SAEs or adverse events. Studies reported a lower risk of moderate exacerbations and adverse events with FDC compared to formoterol; however, larger studies would yield a more precise estimate for these outcomes.
Conflict of interest statement
HN: none known.
SM: none known.
ZS: none known.
KTM: none known.
KNV: none known.
We conducted this systematic review for academic purposes.
Figures





Update of
References
References to studies included in this review
ACLIFORM COPD {published and unpublished data}
-
- Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respiratory Research 2015;16:92. - PMC - PubMed
-
- D'Urzo AD, Singh D, Kerwin E, Lakkis H, Chuecos F, Miquel G. Efficacy and safety of aclidinium/formoterol fixed‐dose combination in patients with moderate to severe airflow obstruction, stratified by severity. European Respiratory Journal 2015;46:PA1499.
-
- EUCTR2011‐001524‐38‐GB. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combinations compared with individual components and placebo when administered to patients with stable chronic obstructive pulmonary disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐001524‐38‐GB Date first received: 5 July 2011.
-
- Jones PW, Singh D, Bateman ED, Korn S, Serra C, Molins E, et al. The effect of aclidinium/formoterol fixed‐dose combination on COPD symptoms and health status in patients with COPD: results from the ACLIFORM/COPD study. American Journal of Respiratory and Critical Care Medicine 2014;189:A3764. [CENTRAL: 1035574; CRS: 4900126000023083]
ACTIVATE {published and unpublished data}
-
- Aymerich JG, Watz H, Beeh KM, Paggiaro P, Moya M, Notari M, et al. Use of the daily PROactive instrument to evaluate physical activity in patients with COPD: results from ACTIVATE. European Respiratory Journal 2017; Vol. 50, issue Suppl 61:PA4964.
-
- EUCTR2014‐005318‐50‐HU. A multiple dose, randomised, double‐blind, placebo controlled, parallel clinical trial to assess the effect of aclidinium bromide/formoterol fumarate fixed‐dose combination on lung hyperinflation, exercise capacity and physical activity in patients with moderate to severe chronic obstructive pulmonary disease (COPD). ACTIVATE. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐005318‐50‐HU Date first received: 2 March 2015.
-
- NCT02424344. Effect of aclidinium/formoterol on lung hyperinflation, exercise capacity and physical activity in moderate to severe COPD patients. clinicaltrials.gov/ct2/show/NCT02424344 Date first received: 23 April 2015.
-
- Watz H, Troosters T, Beeh KM, Aymerich JG, Paggiaro P, Molins E, et al. Effect of aclidinium/formoterol on lung hyperinflation, exercise capacity and physical activity in patients with COPD: results from ACTIVATE: a phase IV study. American Journal of Respiratory and Critical Care Medicine 2017;195:A3593.
AUGMENT COPD {published and unpublished data}
-
- Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respiratory Research 2015;16:92. - PMC - PubMed
-
- D'Urzo A, Mergel V, Leselbaum A, Caracta C. Efficacy and safety of fixed‐dose combination aclidinium bromide/formoterol fumarate in patients with COPD: results from the AUGMENT COPD trial. Chest 2013;144(4):1025A. [EMBASE: 71270077]
-
- D'Urzo A, Rennard S, Kerwin E, Mergel V, He T, Leselbaum A, et al. One‐year efficacy of aclidinium/formoterol fixed‐dose combination in COPD patients: the AUGMENT COPD study. European Respiratory Journal 2014;44(Suppl 58):P286.
-
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The AUGMENT COPD trial: efficacy and safety of a fixed‐dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest 2014;145(3 Suppl):426A. [EMBASE: 71429003; PUBMED: 24638583]
-
- D'Urzo AD, Rennard SI, Kerwin EM, He T, Leselbaum A, Caracta C. Efficacy of fixed‐dose combination aclidinium bromide/formoterol fumarate on bronchodilation over 1 year: AUGMENT COPD extension trial in patients with moderate to severe COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A6006. [CENTRAL: 1035555; CRS: 4900126000023064]
D'Urzo 2017a {published and unpublished data}
-
- D'Urzo A, Rennard S, Kerwin E, Donohue JF, Lei A, Molins E, et al. A randomised double‐blind, placebo‐controlled, long‐term extension study of the efficacy, safety and tolerability of fixed‐dose combinations of aclidinium/formoterol or monotherapy in the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2017;125:39‐48. [PUBMED: 28340861] - PubMed
-
- Make B, Donohue JF, Soong W, Zhong X, Leselbaum A, Caracta C. Efficacy and tolerability of aclidinium bromide/formoterol fumarate fixed‐dose combination in patients with COPD: a one‐year study. European Respiratory Journal 2014;44(Suppl 58):P2413.
-
- NCT01572792. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28‐weeks treatment in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01572792 Date first received: 6 April 2012. [CRS: 4900101000003214]
-
- NCT01572792. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28‐weeks treatment in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01572792 Date first received: 6 April 2012. [CRS: 4900101000003158]
-
- Rennard SI, D'Urzo AD, Donohue JF, He T, Shrestha P, Leselbaum A, et al. Long‐term safety of fixed‐dose combination aclidinium bromide/formoterol fumarate in patients with moderate to severe COPD: the AUGMENT COPD extension trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A6008. [CENTRAL: 1035617; CRS: 4900126000023126]
Donohue 2016 {published and unpublished data}
-
- Make B, Donohue J, Zhong X, Leselbaum A, Caracta C. Long‐term safety of a fixed‐dose combination of aclidinium bromide/formoterol fumarate in patients with stable moderate to severe COPD. Chest 2014;145(3 Suppl):386A. [EMBASE: 71428967; PUBMED: 24638543]
-
- Make BJ, Donohue JF, Soong W, Zhong X, Leselbaum A, Caracta C. Lung function and safety of aclidinium bromide/formoterol fumarate fixed‐dose combination: results of a one‐year trial in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A6010. [CENTRAL: 1035588; CRS: 4900126000023097]
-
- NCT01437540. Safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (LAC). clinicaltrials.gov/ct2/show/NCT01437540 Date first received: 21 September 2011.
NCT00706914 {unpublished data only}
-
- NCT00706914. Comparison of aclidinium bromide and formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00706914 Date first received: 30 June 2008. [CRS: 4900120000000479]
Sliwinski 2010 {published and unpublished data}
-
- EUCTR2007‐004435‐30‐CZ. A randomised, four‐week, placebo‐controlled, double‐blind, six arm parallel group, dose‐finding clinical trial, to assess the efficacy and safety of three different doses of formoterol (6, 12 & 18 µg) combined with the inhaled anticholinergic aclidinium bromide 200 µg, aclidinium bromide 200 µg monotherapy and formoterol 12 µg monotherapy all administered once daily by inhalation via Almirall inhaler in patients with stable moderate to severe chronic obstructive pulmonary disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007‐004435‐30‐CZ Date first received: 10 December 2007. [CRS: 4900120000000461]
-
- NCT00626522. Aclidinium/formoterol fixed combination dose finding study. clinicaltrials.gov/ct2/show/NCT00626522 Date first received: 29 February 2008.
-
- Sliwinski P, Perng D‐W, Chuchalin A, Jones PW. Efficacy and safety of once‐daily aclidinium bromide 200 μg in combination with formoterol in patients with COPD. Thorax 2010;65(Suppl IV):A136. [CENTRAL: 783128; CRS: 4900100000026295; EMBASE: 70325994]
References to studies excluded from this review
ALIGHT‐COPD {unpublished data only}
-
- NCT01078623. Efficacy and safety of two fixed dose combinations of aclidinium bromide with formoterol fumarate (ALIGHT‐COPD). clinicaltrials.gov/ct2/show/NCT01078623 Date first received: 2 March 2010. [CRS: 4900101000003146]
ASTUTE {unpublished data only}
-
- NCT03181880. To evaluate effectiveness of aclidinium bromide/formoterol fumarate dihydrate in chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT03181880 Date first received: 9 June 2017.
EUCTR2007‐003648‐31‐DE {unpublished data only}
-
- EUCTR2007‐003648‐31. A phase IIa, randomised, multicentre, evaluator‐blinded, 4‐way crossover clinical trial to study the pharmacokinetics, safety, tolerability and effects on lung function of one day treatment of formoterol 12 µg qd delivered by two different dry powder inhalers (Aerolizer® and Almirall Inhaler), of the fixed dose combination formoterol 12 µg + aclidinium bromide 200 µg qd delivered by Almirall inhaler, and of formoterol 12 µg bid delivered by Aerolizer®, in moderate to severe chronic obstructive pulmonary disease patients. www.clinicaltrialsregister.eu/ctr‐search/search?query=2007‐003648‐31 Date first received: 21 February 2008. [CRS: 4900120000000469]
Fogarty 2014 {published and unpublished data}
-
- Fogarty C, Ortiz S. Pharmacokinetics, safety, and tolerability of aclidinium/formoterol fixed dose combination via Pressair/Genuair vs formoterol via Foradil aerolizer in patients with moderate to severe COPD. Chest 2014;146(4, Supp 2):45A. [EMBASE: 71780177]
-
- NCT01551888. Pharmacokinetic, safety and tolerability study of aclidinium/formoterol fixed dose combination and formoterol in patients with moderate to severe chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01551888 Date first received: 13 March 2012.
ISRCTN11017699 {unpublished data only}
-
- ISRCTN11017699. Histological, cellular and molecular investigation of steroid responsiveness in chronic obstructive pulmonary disease – the HISTORIC study. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN11017699 Date first received: 15 November 2016.
Kerwin 2013 {published and unpublished data}
-
- EUCTR2009‐015901‐38. Efficacy, safety and tolerability of two fixed‐dose combinations of aclidinium bromide with two doses of formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo all administered twice daily in stable, moderate to severe chronic obstructive pulmonary disease patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐015901‐38‐DE Date first received: 11 November 2009. [CRS: 4900120000000473]
-
- Kerwin E, Lapidus R, Leselbaum A, Ortiz S, Rowe P, Caracta C. Dose‐ranging study of two fixed‐dose combinations of twice‐daily aclidinium bromide plus formoterol in patients with moderate to severe COPD. Chest 2013;144(4):747A. [EMBASE: 71269833]
-
- NCT01049360. Efficacy and safety study of two fixeddose combinations of aclidinium bromide with formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo (LACMD27). clinicaltrials.gov/ct2/show/NCT01049360 Date first received: 14 January 2010.
MUSIC {unpublished data only}
-
- EUCTR2016‐000734‐21‐GB. Investigating the mechanism of inhaled corticosteroids associated pneumonia by longitudinal characterisation of the airway microbiome in patients with severe COPD ‐ microbiome use to stratify use of inhaled corticosteroids: MUSIC. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016‐000734‐21‐GB Date first received: 24 August 2016.
-
- NCT02972476. Microbiome use to stratify use of inhaled corticosteroids: MUSIC trial. clinicaltrials.gov/show/NCT02972476 Date first received: 23 November 2016.
NCT02429765 {unpublished data only}
-
- NCT02429765. Effect of aclidinium/formoterol on night time lung function and morning symptoms in chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT02429765 Date first received: 29 April 2015.
NCT03104634 {unpublished data only}
-
- NCT03104634. The effects of inhaled aclidinium bromide/formoterol fumarate on inspiratory pleural pressures in smokers: a randomised, double‐blind, placebo‐controlled, cross‐over trial. clinicaltrials.gov/ct2/show/NCT03104634 Date first received: 7 April 2017.
NCT03275116 {unpublished data only}
-
- EUCTR2016‐003989‐12‐NL. The effect of twice daily aclidinium bromide/formoterol fumarate 340/12 mcg vs once daily tiotropium 'Respimat' 5 mcg on static and dynamic hyperinflation in patients with COPD during 24 hours (BOTH). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016‐003989‐12‐NL Date first received: 10 May 2017.
-
- NCT03275116. The effect of twice daily aclidinium bromide/formoterol fumarate 340/12 mcg vs once daily tiotropium 'Respimat' 5 mcg on static and dynamic hyperinflation in patients with COPD during 24 hours (BOTH). clinicaltrials.gov/ct2/show/NCT03275116 Date first received: 7 September 2017.
NCT03276078 {unpublished data only}
-
- NCT03276078. A phase IIa, open‐label, repeat‐dose clinical trial to evaluate the pharmacokinetics, safety and tolerability of aclidinium bromide/formoterol fumarate fixed dose combination administered twice‐daily by inhalation in Chinese patients with moderate to severe chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT03276078 Date first received: 8 September 2017.
van der Palen 2013 {published data only}
-
- Palen J, Ginko T, Kroker A, Valk P, Goosens M, Padullés L, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opinion on Drug Delivery 2013;10(8):1023‐31. [EMBASE: 2013461274; PUBMED: 23745954] - PubMed
Vogelmeier 2015 {published and unpublished data}
-
- Beier J, Vogelmeier C, Mroz R, Pascual S, Segarra RM, Lei A, et al. Overall and cardiovascular safety of aclidinium/formoterol fixed‐dose combination versus salmeterol/fluticasone in patients with COPD. European Respiratory Journal 2015;46:PA989. [DOI: 10.1183/13993003.congress-2015.PA989] - DOI
-
- EUCTR2013‐000116‐14‐HU. A randomised, double‐blind, double‐dummy, active‐controlled study evaluating the efficacy, safety and tolerability of twice‐daily aclidinium bromide /formoterol fumarate compared with twice‐daily salmeterol/fluticasone propionate for 24‐weeks treatment in symptomatic patients with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐000116‐14‐HU Date first received: 7 June 2013.
-
- NCT01908140. Study of aclidinium bromide/formoterol fumarate compared with salmeterol/fluticasone propionate in patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01908140 Date first received: 25 July 2013. [CRS: 4900120000000486]
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A‐M, et al. Efficacy of aclidinium/formoterol fixed‐dose combination versus salmeterol/fluticasone in COPD. European Respiratory Journal 2015;46(Suppl 59):PA2960.
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A‐M, et al. The efficacy and safety of aclidinium/formoterol fixed‐dose combination compared with salmeterol/fluticasone in patients with COPD: results from a phase III study. American Journal of Respiratory and Critical Care Medicine 2015;191:A3974.
References to studies awaiting assessment
AMPLIFY {unpublished data only}
-
- EUCTR2015‐005444‐33‐GB. A 24 week treatment, multicentre, randomised, double blinded, double dummy, parallel‐group, clinical trial evaluating the efficacy and safety of aclidinium bromide 400 µg/formoterol fumarate 12 µg fixed‐dose combination BID compared with each monotherapy (aclidinium bromide 400 µg BID and formoterol fumarate 12 µg BID) and tiotropium 18 µg QD when administered to patients with stable chronic obstructive pulmonary disease – AMPLIFY. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐005444‐33‐GB Date first received: 13 April 2016.
-
- NCT02796677. AMPLIFY – D6571C00001 Duaklir USA phase III study. clinicaltrials.gov/show/NCT02796677 2016; Vol. Date first received: 13 June 2016.
References to ongoing studies
AVANT {unpublished data only}
-
- NCT03022097. Study to patients with stable COPD to assess the efficacy and safety of aclidinium bromide/formoterol fumarate (DUAKLIR). clinicaltrials.gov/show/NCT03022097 Date first received: 16 January 2017.
Additional references
Almirall
-
- Almirall. Clinical trials registry and results. www.almirall.com/en/about‐almirall/research‐and‐development/clinical‐trials (accessed 26 January 2015).
Appleton 2006
AstraZeneca
-
- AstraZeneca. AstraZeneca Clinical Trials. astrazenecagrouptrials.pharmacm.com/ST/Submission/Search (accessed prior to 19 July 2018).
ATS/ERS 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. - PubMed
Barnes 2000
-
- Barnes PJ. Chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(4):269‐80. [PUBMED: 10911010] - PubMed
Bateman 2015
-
- Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respiratory Research 2015;16:92. [PUBMED: 26233481] - PMC - PubMed
Bauer 2013
-
- Bauer CM, Morissette MC, Stampfli MR. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest 2013;143(1):196‐206. [PUBMED: 23276842] - PubMed
Beier 2009
-
- Beier J, Beeh KM, Brookman L, Peachey G, Hmissi A, Pascoe S. Bronchodilator effects of indacaterol and formoterol in patients with COPD. Pulmonary Pharmacology and Therapeutics 2009;22(6):492‐6. [PUBMED: 19465142] - PubMed
Beier 2011
Berger 2008
-
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102:173‐88. - PubMed
Bruce 2000
Calzetta 2017a
Calzetta 2017b
-
- Calzetta L, Ora J, Cavalli F, Rogliani P, O'Donnell DE, Cazzola M. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: a pair‐wise and network meta‐analysis. Respiratory Medicine 2017;129:189‐98. [PUBMED: 28732830] - PubMed
Capel 2018
-
- Capel M, Mareque M, Alvarez CJ, Lindner L, Oyaguez I. Cost‐effectiveness of fixed‐dose combinations therapies for chronic obstructive pulmonary disease treatment. Clinical Drug Investigation 2018;38(7):611‐20. [PUBMED: 29654555] - PubMed
Cazzola 2010
-
- Cazzola M, Molimard M. The scientific rationale for combining long‐acting beta2‐agonists and muscarinic antagonists in COPD. Pulmonary Pharmacology and Therapeutics 2010;23(4):257‐67. [PUBMED: 20381630] - PubMed
Cazzola 2013
-
- Cazzola M, Rogliani P, Matera MG. Aclidinium bromide/formoterol fumarate fixed‐dose combination for the treatment of chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2013;14(6):775‐81. [PUBMED: 23472632] - PubMed
Chapman 2006
-
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. European Respiratory Journal 2006;27(1):188‐207. [PUBMED: 16387952] - PubMed
Chong 2012
Cosio 2009
-
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. New England Journal of Medicine 2009;360(23):2445‐54. [PUBMED: 19494220] - PubMed
Criner 2015
-
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015; Vol. 147, issue 4:894‐942. [PUBMED: 25321320] - PMC - PubMed
D'Urzo 2017b
de Miguel‐Diez 2014
-
- Miguel‐Diez J, Jimenez‐Garcia R. Considerations for new dual‐acting bronchodilator treatments for chronic obstructive pulmonary disease. Expert Opinion on Investigational Drugs 2014; Vol. 23, issue 4:453‐6. [PUBMED: 24392807] - PubMed
Di Marco 2017
-
- Marco F, Santus P, Scichilone N, Solidoro P, Contoli M, Braido F, et al. Symptom variability and control in COPD: advantages of dual bronchodilation therapy. Respiratory Medicine 2017;125:49‐56. [PUBMED: 28340862] - PubMed
FDA
-
- FDA. United States Food and Drug Administration. www.fda.gov/ (accessed 26 January 2015).
FDA 2011
-
- Food, Drug Administration. FDA approved drug products: Arcapta neohaler (indacaterol inhalation powder). www.accessdata.fda.gov/drugsatfda_docs/label/2012/022383s002lbl.pdf (accessed 24 November 2014).
FDA 2012
-
- Food, Drug Administration. Drug approval package; Tudorza Pressair (aclidinium bromide) inhalation powder. www.accessdata.fda.gov/drugsatfda_docs/label/.../202450s000lbl.pdf (accessed 15 November 2014).
FDA 2013a
-
- Food, Drug Administration. FDA approved drug products: Breo Ellipta (fluticasone furoate and vilanterol inhalation powder). www.accessdata.fda.gov/drugsatfda_docs/label/2013/204275s000lbl.pdf (accessed 24 November 2014).
FDA 2013b
-
- Food, Drug Administration. Drug approval package: Ellipta (umeclidinium /vilanterol) powder for inhalation. www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203975Orig1s000TOC.cfm (accessed 2 December 2014).
Ferguson 2003
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
Forest Pharmaceuticals
-
- Forest Pharmaceuticals, Inc. Research and Development. www.frx.com/Research (accessed 26 January 2015).
Gershon 2013
-
- Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, et al. Cardiovascular safety of inhaled long‐acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Internal Medicine 2013;173(13):1175‐84. - PubMed
GOLD 2018
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2018 report). www.goldcopd.org/ (accessed 11 February 2018).
Hagstad 2014
-
- Hagstad S, Bjerg A, Ekerljung L, Backman H, Lindberg A, Ronmark E, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest 2014;145(6):1298‐304. [PUBMED: 24356778] - PubMed
Hanania 2013
-
- Hanania NA. Evaluating the safety of COPD medications: an evidence‐based review. Chest 2013;144(4):1357‐67. [PUBMED: 24081347] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnson 1998
-
- Johnson M. The beta‐adrenoceptor. American Journal of Respiratory and Critical Care Medicine 1998;158:S146‐53. - PubMed
Karakiulakis 2012
-
- Karakiulakis G, Roth M. Muscarinic receptors and their antagonists in COPD: anti‐inflammatory and anti‐remodeling effects. www.ncbi.nlm.nih.gov/pmc/articles/PMC3512336/ (accessed 16 November 2014).
Karner 2012
Kew 2013
Lal 2017
-
- Lal C, Strange C. A review of current and developing fixed‐dose LABA/LAMA combinations for treating COPD. Expert Opinion on Pharmacotherapy 2017;18(17):1833‐43. [PUBMED: 29115881] - PubMed
Maltais 2012
-
- Maltais F, Milot J. The potential for aclidinium bromide, a new anticholinergic, in the management of chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2012;6(6):345‐61. - PubMed
Mannino 2007
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370(9589):765‐73. [PUBMED: 17765526] - PubMed
Marchetti 2013
-
- Marchetti N, Criner GJ, Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest 2013;143(5):1444‐54. [PUBMED: 23648908] - PubMed
Matera 2014
-
- Matera MG, Rogliani P, Cazzola M. Muscarinic receptor antagonists for the treatment of chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2014;15(7):961‐77. - PubMed
Medic 2016
Miravitlles 2016
-
- Miravitlles M, Chapman KR, Chuecos F, Ribera A, Gil EG. The efficacy of aclidinium/formoterol on lung function and symptoms in patients with COPD categorised by symptom status: a pooled analysis. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:2041‐53. [PUBMED: 27621610] - PMC - PubMed
Moulton 2011
Mullerova 2015
-
- Mullerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha J, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest 2015; Vol. 147, issue 4:999‐1007. [PUBMED: 25356881] - PubMed
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] - DOI
Nannini 2013a
Nannini 2013b
-
- Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006826.pub2] - DOI - PMC - PubMed
Ni 2014
Ni 2017
NICE 2010
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update). www.nice.org.uk/guidance/cg101/resources/guidance‐chronic‐obstructive‐pu... (accessed 30 October 2014).
Oba 2016
-
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long‐acting beta‐agonist/long‐acting muscarinic antagonist combinations in COPD: a network meta‐analysis. Thorax 2016;71(1):15‐25. [PUBMED: 26490732] - PubMed
PubMed
-
- PubMed. US National Library of Medicine, National Institutes of Health database. www.ncbi.nlm.nih.gov/pubmed (accessed 30 October 2014).
Raherison 2009
-
- Raherison C, Girodet PO. Epidemiology of COPD. European Respiratory Review 2009;18(114):213‐21. [PUBMED: 20956146] - PubMed
Ramos 2016
-
- Ramos M, Haughney J, Henry N, Lindner L, Lamotte M. Cost versus utility of aclidinium bromide 400 microg plus formoterol fumarate dihydrate 12 microg compared to aclidinium bromide 400 microg alone in the management of moderate‐to‐severe COPD. ClinicoEconomics and Outcomes Research 2016;8:445‐56. [PUBMED: 27672337] - PMC - PubMed
Rennard 2006
-
- Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367(9518):1216‐9. [PUBMED: 16631861] - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera 2008
-
- Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez‐Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. International Journal of Tuberculosis and Lung Disease 2008;12(8):972‐7. [PUBMED: 18647460] - PubMed
Rodrigo 2008
-
- Rodrigo GJ, Nannini LJ, Rodríguez‐Roisin R. Safety of long acting beta‐agonists in stable COPD: a systematic review. Chest 2008;133(5):1079‐87. - PubMed
Rodrigo 2017
Sims 2011
Spencer 2011
Sutherland 2004
-
- Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 2004;350(26):2689‐97. [PUBMED: 15215485] - PubMed
Tashkin 2004
-
- Tashkin DP, Cooper CB. The role of long‐acting bronchodilators in the management of stable COPD. Chest 2004;125(1):249‐59. [PUBMED: 14718448] - PubMed
Tashkin 2008
-
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, et al. Bronchodilator responsiveness in patients with COPD. European Respiratory Journal 2008;31(4):742‐50. [PUBMED: 18256071] - PubMed
Tashkin 2013
TSANZ 2018
-
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al. The COPD‐X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2018. Version 2.53, March 2018. www.copdx.org.au/ (accessed 10 July 2018).
Ulrik 2012
van der Molen 2012
Welte 2015
WHO
-
- Diagnosis of COPD. http://www.who.int/respiratory/copd/diagnosis/en/.
WHO ICTRP
-
- World Health Organization. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed 30 October 2014).
Zafar 2014
-
- Zafar MA, Droege C, Foertsch M, Panos RJ. Update on ultra‐long‐acting beta agonists in chronic obstructive pulmonary disease. Expert Opinion on Investigational Drugs 2014;23(12):1687‐701. [PUBMED: 25139313] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

