Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides
- PMID: 30536739
- PMCID: PMC6489501
- DOI: 10.1002/anie.201812894
Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides
Abstract
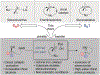
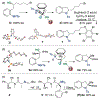
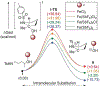
The direct, catalytic substitution of unactivated alcohols remains an undeveloped area of organic synthesis. Moreover, catalytic activation of this difficult electrophile with predictable stereo-outcomes presents an even more formidable challenge. Described herein is a simple iron-based catalyst system which provides the mild, direct conversion of secondary and tertiary alcohols to sulfonamides. Starting from enantioenriched alcohols, the intramolecular variant proceeds with stereoinversion to produce enantioenriched 2- and 2,2-subsituted pyrrolidines and indolines, without prior derivatization of the alcohol or solvolytic conditions.
Keywords: alcohol substitution; asymmetric synthesis; indoline; iron; sulfonamides.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
References
-
- Cowdrey WA, Hughes ED, Ingold CK, Masterman S,Scott AD, J. Chem. Soc 1937, 1252;
- Xie J, Hase WL, Science 2016, 352, 32–33. - PubMed
-
- Carey FA, Sundberg RJ, Advanced Organic Chemistry, 5th ed., Springer, New York, 2007.
-
- Pronin SV, Reiher CA, Shenvi RA, Nature 2013, 501, 195–199. - PubMed
-
- Bryan MC, Dunn PJ, Entwistle D, Gallou F, Koenig SG,Hayler JD, Hickey MR, Hughes S, Kopach ME, Moine G, Richardson P, Roschangar F, Steven A, Weiberth FJ, Green Chem. 2018, 20, 5082–5103.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

