Trial Versus No Trial of Spinal Cord Stimulation for Chronic Neuropathic Pain: Cost Analysis in United Kingdom National Health Service
- PMID: 30536992
- PMCID: PMC6590634
- DOI: 10.1111/ner.12898
Trial Versus No Trial of Spinal Cord Stimulation for Chronic Neuropathic Pain: Cost Analysis in United Kingdom National Health Service
Abstract
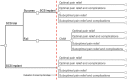
Objectives: The aim of the current project was to evaluate the spinal cord stimulation (SCS) screening trial success rate threshold to obtain the same cost impact across two identical sets of patients following either a prolonged screening trial prior to implantation strategy or a full implant without a screening trial.
Materials and methods: A cost impact analysis was carried out from a health care perspective and considered trial to implant rates reported in the literature. Items of resource use were costed using national averages obtained from the National Health Service (NHS) reference cost data base. Cost components were added up to derive total patient level costs for the NHS. Only the costs associated with the screening trial procedures and devices were considered.
Results: The most conservative of our estimates suggest that a failure rate of less than 15% is cost saving to the NHS. A failure rate as high as 45% can also be cost saving if the less expensive nonrechargeable SCS devices are used. All the thresholds observed represent a considerably higher screening failure rate than that reported in the latest randomized controlled trials (RCTs) of SCS. A trial to implant ratio of 91.6% could represent savings between £16,715 (upper bound 95% CI of rechargeable implantable pulse generator [IPG] cost) and £246,661 (lower bound 95% CI of nonrechargeable IPG cost) per each 100 patients by adopting an implantation only strategy.
Conclusions: Considerable savings could be obtained by adopting an implantation strategy without a screening trial. It is plausible that accounting for other factors, such as complications that can occur with a screening trial, additional savings could be achieved by choosing a straight to implant treatment strategy. Nevertheless, additional evidence is warranted to support this claim.
Keywords: Cost comparison; screening trial; spinal cord stimulation.
© 2018 The Authors. Neuromodulation: Technology at the Neural Interface published by Wiley Periodicals, Inc. on behalf of International Neuromodulation Society.
Figures
References
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. - PubMed
-
- Moody A. Diabetes and hyperglycaemia In: Craig R, Mindell J, editors. Health survey for England 2011, health, social care and lifestyles. London: The Health and Social Care Information Centre, 2012; p. 1–28.
-
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–662. - PubMed
-
- Kumar K, Taylor RS, Jacques L et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179–188. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources