How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study
- PMID: 30537226
- PMCID: PMC6590461
- DOI: 10.1111/dom.13612
How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study
Abstract
Aims: Enrollment criteria vary substantially among cardiovascular outcome trials (CVOTs) of sodium-glucose cotransporter-2 inhibitors (SGLT-2is), which impacts the relationship between a trial population and the general type 2 diabetes (T2D) population. The aim of this study was to evaluate the representativeness of four SGLT-2i CVOTs of a general T2D population.
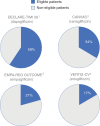
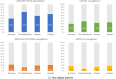
Methods: T2D patients from Germany, The Netherlands, Norway and Sweden were included in the study. Given the available data per country, key inclusion and exclusion criteria were defined by diagnoses, procedures and drug treatments to facilitate comparability among countries. Representativeness was determined by dividing the number of patients fulfilling the key enrolment criteria of each CVOT (CANVAS, DECLARE-TIMI 58, EMPA-REG OUTCOME, VERTIS-CV) by the total T2D population.
Results: In 2015, a total T2D population of 803 836 patients was identified in Germany (n = 239 485), in The Netherlands (n = 36 213), in Norway (n = 149 782) and in Sweden (n = 378 356). These populations showed a 25% to 44% cardiovascular (CV) disease baseline prevalence and high CV-preventive drug use (>80%). The general T2D population had less prevalent CV disease and patients were slightly older than those included in the CVOTs. The DECLARE-TIMI 58 trial had the highest representativeness, 59% compared to the general T2D population, and this representativeness was almost 2-, 3- and 4-fold higher compared to the CANVAS (34%), EMPA-REG OUTCOME (21%) and VERTIS-CV (17%) trials, respectively.
Conclusions: In large T2D populations within Europe, consistent patterns of representativeness of CVOTs were found when applying the main enrolment criteria. The DECLARE-TMI 58 trial had the highest representativeness, indicating that it included and examined patients who are most representative of the general T2D patients in the studied countries.
Keywords: SGLT2 inhibitors; cardiovascular disease; cardiovascular outcome trial; observational study; representativeness.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
All authors participated in the research design. M. T., J. G. K. and E. G. performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility for the decision to submit for publication. All authors are guarantors of the manuscript.
Figures
References
-
- Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59:1692‐1701. - PubMed
-
- Cannon CP, McGuire D, Pratley R, et al. Design and baseline characteristics of the evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial (VERTIS‐CV). Am Heart J. 2018;206:11‐23. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. - PubMed
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

