Prevention of Early Postoperative Decline (PEaPoD): protocol for a randomized, controlled feasibility trial
- PMID: 30537982
- PMCID: PMC6290549
- DOI: 10.1186/s13063-018-3063-z
Prevention of Early Postoperative Decline (PEaPoD): protocol for a randomized, controlled feasibility trial
Abstract
Background: Delirium is associated with a significantly increased risk of postoperative morbidity and mortality. Furthermore, delirium has been associated with an increased risk of prolonged cognitive deficits and accelerated long-term cognitive decline. To date, experimental interventions for delirium have mainly focused on alternative pharmacologic and behavioral strategies in the postoperative period. Few studies have examined whether proactive strategies started before surgery can prevent delirium or reduce its sequelae. Neurocognitive training programs such as Lumosity have been shown to be effective in increasing cognitive performance in both elderly healthy volunteers and patients suffering from a myriad of acute and chronic medical conditions. When initiated in the preoperative period, such training programs may serve as interesting and novel patient-led interventions for the prevention of delirium and postoperative cognitive decline (POCD). We hypothesize that perioperative neurocognitive training is feasible in the older cardiac surgical population and are testing this hypothesis using a randomized controlled design.
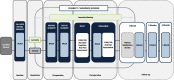
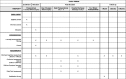
Methods: The Prevention of Early Postoperative Decline (PEaPoD) study is a randomized, controlled trial with a target enrollment of 45 elderly cardiac surgical patients. Subjects will be randomized in a 1:1 ratio to undergo either at least 10 days of preoperative neurocognitive training, continued for 4 weeks postoperatively, or usual care control. The primary outcome, feasibility, will be assessed by study recruitment and adherence to protocol. Secondary outcomes will include potential differences in the incidence of postoperative in-hospital delirium and POCD up to 6 months, as determined by the Confusion Assessment Method and the Montreal Cognitive Assessment.
Discussion: PEaPoD will be the first trial investigating the use of perioperative cognitive training to potentially reduce delirium and POCD in the cardiac surgical population. Information gleaned from this feasibility study will prove valuable in designing future efficacy studies aimed at determining whether this low-risk, patient-led intervention can reduce serious postoperative morbidity.
Trial registration: ClinicalTrials.gov, NCT02908464 . Registered on 21 September 2016.
Keywords: Cardiac surgery; Confusion Assessment Method; Delirium; Montreal Cognitive Assessment; Neurocognitive training; Postoperative cognitive decline.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Committee on Clinical Investigations at BIDMC (IRB Protocol #2016P000145). Written informed consent is obtained for all subjects prior to initiation of study procedures. Written informed consent is obtained by the principal investigator and/or research team members given specific training to gain consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical