HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer
- PMID: 30537988
- PMCID: PMC6288935
- DOI: 10.1186/s13148-018-0589-6
HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer
Abstract
Background: B7 homolog 1 (B7-H1) overexpression on tumor cells is an important mechanism of immune evasion in gastric cancer (GC). Elucidation of the regulation of B7-H1 expression is urgently required to guide B7-H1-targeted cancer therapy. Interferon gamma (IFN-γ) is thought to be the main driving force behind B7-H1 expression, and epigenetic factors including histone acetylation are recently linked to the process. Here, we investigated the potential role of histone deacetylase (HDAC) in IFN-γ-induced B7-H1 expression in GC. The effect of Vorinostat (SAHA), a small molecular inhibitor of HDAC, on tumor growth and B7-H1 expression in a mouse GC model was also evaluated.
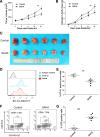
Results: RNA-seq data from The Cancer Genome Atlas revealed that expression of B7-H1, HDAC1-3, 6-8, and 10 and SIRT1, 3, 5, and 6 was higher, and expression of HDAC5 and SIRT4 was lower in GC compared to that in normal gastric tissues; that HDAC3 and HDAC1 expression level significantly correlated with B7-H1 in GC with a respective r value of 0.42 (p < 0.001) and 0.21 (p < 0.001). HDAC inhibitor (Trichostatin A, SAHA, and sodium butyrate) pretreatment suppressed IFN-γ-induced B7-H1 expression on HGC-27 cells. HDAC1 and HDAC3 gene knockdown had the same effect. SAHA pretreatment or HDAC knockdown resulted in impaired IFN-γ signaling, demonstrated by the reduction of JAK2, p-JAK1, p-JAK2, and p-STAT1 expression and inefficient STAT1 nuclear translocation. Furthermore, SAHA pretreatment compromised IFN-γ-induced upregulation of histone H3 lysine 9 acetylation level in B7-H1 gene promoter. In the grafted mouse GC model, SAHA treatment suppressed tumor growth, inhibited B7-H1 expression, and elevated the percentage of tumor-infiltrating CD8+ T cells.
Conclusion: HDAC is indispensable for IFN-γ-induced B7-H1 in GC. The study suggests the possibility of targeting B7-H1 using small molecular HDAC inhibitors for cancer treatment.
Keywords: B7-H1; Gastric cancer; HDAC; IFN-γ; Immune evasion.
Conflict of interest statement
Ethics approval and consent to participate
All participating individuals were informed of the study and provided their written consent. The study protocols were approved by the Ethics Committees of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. All animal experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. Animal experimental protocols were approved by the Animal Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
-
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2017.1747. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

