Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial
- PMID: 30538086
- PMCID: PMC6305881
- DOI: 10.2196/10802
Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial
Abstract
Background: Vital signs monitoring is a universal tool for the detection of postoperative complications; however, unwell patients can be missed between traditional observation rounds. New remote monitoring technologies promise to convey the benefits of continuous monitoring to patients in general wards.
Objective: The aim of this pilot study was to evaluate whether continuous remote vital signs monitoring is a practical and acceptable way of monitoring surgical patients and to optimize the delivery of a definitive trial.
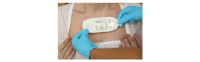
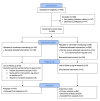
Methods: We performed a prospective, cluster-randomized, parallel-group, unblinded, controlled pilot study. Patients admitted to 2 surgical wards at a large tertiary hospital received either continuous and intermittent vital signs monitoring or intermittent monitoring alone using an early warning score system. Continuous monitoring was provided by a wireless patch, worn on the patient's chest, with data transmitted wirelessly every 2 minutes to a central monitoring station or a mobile device carried by the patient's nurse. The primary outcome measure was time to administration of antibiotics in sepsis. The secondary outcome measures included the length of hospital stay, 30-day readmission rate, mortality, and patient acceptability.
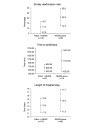
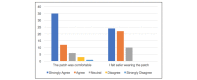
Results: Overall, 226 patients were randomized between January and June 2017. Of 226 patients, 140 were randomized to continuous remote monitoring and 86 to intermittent monitoring alone. On average, patients receiving continuous monitoring were administered antibiotics faster after evidence of sepsis (626 minutes, n=22, 95% CI 431.7-820.3 minutes vs 1012.8 minutes, n=12, 95% CI 425.0-1600.6 minutes), had a shorter average length of hospital stay (13.3 days, 95% CI 11.3-15.3 days vs 14.6 days, 95% CI 11.5-17.7 days), and were less likely to require readmission within 30 days of discharge (11.4%, 95% CI 6.16-16.7 vs 20.9%, 95% CI 12.3-29.5). Wide CIs suggest these differences are not statistically significant. Patients found the monitoring device to be acceptable in terms of comfort and perceived an enhanced sense of safety, despite 24% discontinuing the intervention early.
Conclusions: Remote continuous vital signs monitoring on surgical wards is practical and acceptable to patients. Large, well-controlled studies in high-risk populations are required to determine whether the observed trends translate into a significant benefit for continuous over intermittent monitoring.
Trial registration: International Standard Randomised Controlled Trial Number ISRCTN60999823; http://www.isrctn.com /ISRCTN60999823 (Archived by WebCite at http://www.webcitation.org/73ikP6OQz).
Keywords: general surgery; monitoring; physiological; randomized controlled trial; vital signs.
©Candice Downey, Rebecca Randell, Julia Brown, David G Jayne. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 11.12.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Comment in
-
Practical Considerations and Successful Implementation of Vital Signs Monitoring. Comment on "Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial".J Med Internet Res. 2021 Mar 11;23(3):e14042. doi: 10.2196/14042. J Med Internet Res. 2021. PMID: 33704079 Free PMC article. No abstract available.
References
-
- Bartels K, Karhausen J, Clambey ET, Grenz A, Eltzschig HK. Perioperative organ injury. Anesthesiology. 2013 Dec;119(6):1474–89. doi: 10.1097/ALN.0000000000000022. http://europepmc.org/abstract/MED/24126264 - DOI - PMC - PubMed
-
- International Surgical Outcomes Study group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016 Oct 31;117(5):601–609. doi: 10.1093/bja/aew316. https://linkinghub.elsevier.com/retrieve/pii/S0007-0912(17)30018-1 S0007-0912(17)30018-1 - DOI - PMC - PubMed
-
- Michard F, Gan T J, Kehlet H. Digital innovations and emerging technologies for enhanced recovery programmes. Br J Anaesth. 2017 Jul 01;119(1):31–39. doi: 10.1093/bja/aex140. https://linkinghub.elsevier.com/retrieve/pii/S0007-0912(17)33736-4 S0007-0912(17)33736-4 - DOI - PubMed
-
- Goldhill DR, McNarry AF. Physiological abnormalities in early warning scores are related to mortality in adult inpatients. Br J Anaesth. 2004 Jun;92(6):882–4. doi: 10.1093/bja/aeh113. https://linkinghub.elsevier.com/retrieve/pii/S0007-0912(17)35567-8 S0007-0912(17)35567-8 - DOI - PubMed
-
- Ridley S. The recognition and early management of critical illness. Ann R Coll Surg Engl. 2005 Sep;87(5):315–22. doi: 10.1308/003588405X60669. http://europepmc.org/abstract/MED/16176687 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

