GATA2 deficiency and human hematopoietic development modeled using induced pluripotent stem cells
- PMID: 30538114
- PMCID: PMC6290105
- DOI: 10.1182/bloodadvances.2018017137
GATA2 deficiency and human hematopoietic development modeled using induced pluripotent stem cells
Abstract
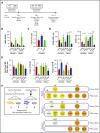
GATA2 deficiency is an inherited or sporadic genetic disorder characterized by distinct cellular deficiency, bone marrow failure, various infections, lymphedema, pulmonary alveolar proteinosis, and predisposition to myeloid malignancies resulting from heterozygous loss-of-function mutations in the GATA2 gene. How heterozygous GATA2 mutations affect human hematopoietic development or cause characteristic cellular deficiency and eventual hypoplastic myelodysplastic syndrome or leukemia is not fully understood. We used induced pluripotent stem cells (iPSCs) to study hematopoietic development in the setting of GATA2 deficiency. We performed hematopoietic differentiation using iPSC derived from patients with GATA2 deficiency and examined their ability to commit to mesoderm, hemogenic endothelial precursors (HEPs), hematopoietic stem progenitor cells, and natural killer (NK) cells. Patient-derived iPSC, either derived from fibroblasts/marrow stromal cells or peripheral blood mononuclear cells, did not show significant defects in committing to mesoderm, HEP, hematopoietic stem progenitor, or NK cells. However, HEP derived from GATA2-mutant iPSC showed impaired maturation toward hematopoietic lineages. Hematopoietic differentiation was nearly abolished from homozygous GATA2 knockout (KO) iPSC lines and markedly reduced in heterozygous KO lines compared with isogenic controls. On the other hand, correction of the mutated GATA2 allele in patient-specific iPSC did not alter hematopoietic development consistently in our model. GATA2 deficiency usually manifests within the first decade of life. Newborn and infant hematopoiesis appears to be grossly intact; therefore, our iPSC model indeed may resemble the disease phenotype, suggesting that other genetic, epigenetic, or environmental factors may contribute to bone marrow failure in these patients following birth. However, heterogeneity of PSC-based models and limitations of in vitro differentiation protocol may limit the possibility to detect subtle cellular phenotypes.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

