Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance
- PMID: 30538186
- PMCID: PMC6299484
- DOI: 10.1128/mBio.02391-18
Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance
Abstract
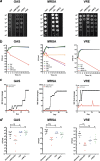
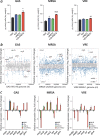
The World Health Organization reports that antibiotic-resistant pathogens represent an imminent global health disaster for the 21st century. Gram-positive superbugs threaten to breach last-line antibiotic treatment, and the pharmaceutical industry antibiotic development pipeline is waning. Here we report the synergy between ionophore-induced physiological stress in Gram-positive bacteria and antibiotic treatment. PBT2 is a safe-for-human-use zinc ionophore that has progressed to phase 2 clinical trials for Alzheimer's and Huntington's disease treatment. In combination with zinc, PBT2 exhibits antibacterial activity and disrupts cellular homeostasis in erythromycin-resistant group A Streptococcus (GAS), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE). We were unable to select for mutants resistant to PBT2-zinc treatment. While ineffective alone against resistant bacteria, several clinically relevant antibiotics act synergistically with PBT2-zinc to enhance killing of these Gram-positive pathogens. These data represent a new paradigm whereby disruption of bacterial metal homeostasis reverses antibiotic-resistant phenotypes in a number of priority human bacterial pathogens.IMPORTANCE The rise of bacterial antibiotic resistance coupled with a reduction in new antibiotic development has placed significant burdens on global health care. Resistant bacterial pathogens such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus are leading causes of community- and hospital-acquired infection and present a significant clinical challenge. These pathogens have acquired resistance to broad classes of antimicrobials. Furthermore, Streptococcus pyogenes, a significant disease agent among Indigenous Australians, has now acquired resistance to several antibiotic classes. With a rise in antibiotic resistance and reduction in new antibiotic discovery, it is imperative to investigate alternative therapeutic regimens that complement the use of current antibiotic treatment strategies. As stated by the WHO Director-General, "On current trends, common diseases may become untreatable. Doctors facing patients will have to say, Sorry, there is nothing I can do for you."
Keywords: Enterococcus faecium; Staphylococcus aureus; Streptococcus pyogenes; antibiotic resistance.
Copyright © 2018 Bohlmann et al.
Figures

References
-
- World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET....
-
- EARS-Net. 2018. European Antimicrobial Resistance Surveillance Network (EARS-Net). http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Pages/index.aspx.
-
- Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/biggest_threats.html.
-
- Center for Disease Dynamics, Economics & Policy. 2015. The state of the world’s antibiotics, 2015. Center for Disease Dynmics, Economics & Policy, Washington, DC: https://cddep.org/sites/default/files/swa_2015_final.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
