Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis
- PMID: 30538296
- PMCID: PMC6484510
- DOI: 10.1038/s41388-018-0613-5
Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis
Abstract
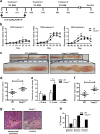
Murine inflammatory caspase-11 has an important role in intestinal epithelial inflammation and barrier function. Activation of the non-canonical inflammasome, mediated by caspase-11, serves as a regulatory pathway for the production of the pro-inflammatory cytokines IL-1β and IL-18, and has a key role in pyroptotic cell death. We have previously demonstrated a protective role for caspase-11 during dextran sulphate sodium (DSS)-induced colitis, however the importance of caspase-11 during colorectal tumour development remains unclear. Here, we show that Casp11-/- mice are highly susceptible to the azoxymethane (AOM)-DSS model of colitis-associated cancer (CAC), compared to their wild type (WT) littermates. We show that deficient IL-18 production occurs at initial inflammation stages of disease, and that IL-1β production is more significantly impaired in Casp11-/- colons during established CAC. We identify defective STAT1 activation in Casp11-/- colons during disease progression, and show that IL-1β signalling induces caspase-11 expression and STAT1 activation in primary murine macrophages and intestinal epithelial cells. These findings uncover an anti-tumour role for the caspase-11 and the non-canonical inflammasome during CAC, and suggest a critical role for caspase-11, linking IL-1β and STAT1 signalling pathways.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- CF-2015-0061P/Enterprise Ireland/International
- CF-2015-0061P/Enterprise Ireland/International
- CF-2015-0061P/Enterprise Ireland/International
- 721906/EC | Horizon 2020 (Horizon 2020 - Research and Innovation Framework Programme)/International
- 721906/EC | Horizon 2020 (Horizon 2020 - Research and Innovation Framework Programme)/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

