Seroprevalence, isolation, molecular detection and genetic diversity of Toxoplasma gondii from small ruminants in Egypt
- PMID: 30538350
- PMCID: PMC6261144
- DOI: 10.1007/s12639-018-1029-4
Seroprevalence, isolation, molecular detection and genetic diversity of Toxoplasma gondii from small ruminants in Egypt
Abstract
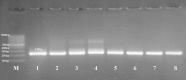
Toxoplasmosis is an infectious zoonotic disease caused by protozoan Toxoplasma gondii. Detection of T. gondii infection with touchy and particular strategies is a key advance to control and prevent toxoplasmosis. Genotyping can explain the virulence, epidemiology and setting up new methodologies for diagnosis and control in human and animals. The point of this study was to assess the seroprevalence of T. gondii in sheep and goat in Egypt and to comprehend the genetic variety of T. gondii isolates circling in Egypt. Blood samples were gathered from 113 ewes and 95 she-goats from three Egyptian governorates (Cairo, Giza and Al-Sharkia). Also blood and tissue samples were gathered from 193 sheep and 51 goats from Cairo and Giza abattoirs. All samples were assayed serologically utilizing ELISA and OnSite Toxo IgG/IgM Rapid test cassettes (OTRT) tests and the tissue samples of the seropositive animals were digested and microscopically examined then bio-assayed in mice as viability test. All the T. gondii isolates undergo molecular identification using PCR and genotyped utilizing nPCR/RFLP analysis of SAG2 gene. The total seropositivity of live sheep and goat was 47.15 and 39.2% utilizing ELISA and OTRT respectively. Concerning abattoirs, seropositivity, positive microscopic examination, mice viability from sheep samples were 47.1%, 37.3% and 44.1% respectively while that of goats were 45.5%, 33.3% and 48.6% respectively. Eighteen T. gondii isolates were affirmed utilizing PCR. Genotyping confirmed 10 isolates (55.5%) as type II, 6 (33.3%) as type III and 2 (11.1%) as atypical genotypes. Type II and III are the genotypes mostly circling among small ruminants in Egypt and this is most significance for the public health in Egypt.
Keywords: Egypt; Genotyping; Goat; SAG2 gene; Sheep; Toxoplasma gondii.
Conflict of interest statement
We confirm that there are no known conflicts of interest associated with this publication. The authors also declare that the trials conducted in this work fulfill with the existing country laws.The study was approved Ethically by the Medical Research Ethical Committee, National Research Centre, Egypt under registration number 1-2 /0- 2 -1-0.2012.
Figures





References
-
- Abd El-Razik KA, El Fadally HA, Barakat AMA, Abu Elnaga ASM. Zoonotic hazards T. gondii viable cysts in ready to eat Egyptian meat-meals. World J Med Sci. 2014;11:510–517.
-
- Abdel-Hameed DM, Hassanein OM. Genotyping of Toxoplasma gondii strains from female patients with toxoplasmosis. J Egypt Soc Parasitol. 2008;38:511–520. - PubMed
-
- Abdel-Rahman MAM, EL-Manyawe SM, Khateib AM, Saba S. Occurrence of Toxoplasma antibodies in caprine milk and serum in Egypt. Assiut Vet Med J. 2012;58:145–152.
-
- Ahmed HA, Shafik SM, Ali MEM, Elghamry ST, Ahmed AA. Molecular detection of Toxoplasma gondii DNA in milk and risk factors analysis of seroprevalence in pregnant women at Sharkia. Egypt Vet World. 2014;7:594–600. doi: 10.14202/vetworld.2014.594-600. - DOI
LinkOut - more resources
Full Text Sources
