Bone Environment Influences Irreversible Adhesion of a Methicillin-Susceptible Staphylococcus aureus Strain
- PMID: 30538688
- PMCID: PMC6277558
- DOI: 10.3389/fmicb.2018.02865
Bone Environment Influences Irreversible Adhesion of a Methicillin-Susceptible Staphylococcus aureus Strain
Abstract
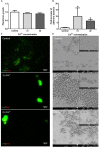
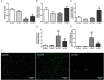
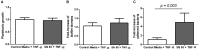
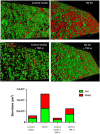
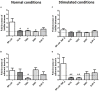
Prosthesis and joint infections are an important threat in public health, especially due to the development of bacterial biofilms and their high resistance to antimicrobials. Biofilm-associated infections increase mortality and morbidity rates as well as hospitalization costs. Prevention is the best strategy for this serious issue, so there is an urgent need to understand the signals that could induce irreversible bacterial adhesion on a prosthesis. In this context, we investigated the influence of the bone environment on surface adhesion by a methicillin-susceptible Staphylococcus aureus strain. Using static and dynamic biofilm models, we tested various bone environment factors and showed that the presence of Mg2+, lack of oxygen, and starvation each increased bacterial adhesion. It was observed that human osteoblast-like cell culture supernatants, which contain secreted components that would be found in the bone environment, increased bacterial adhesion capacity by 2-fold (p = 0.015) compared to the medium control. Moreover, supernatants from osteoblast-like cells stimulated with TNF-α to mimic inflammatory conditions increased bacterial adhesion by almost 5-fold (p = 0.003) without impacting on the overall biomass. Interestingly, the effect of osteoblast-like cell supernatants on bacterial adhesion could be counteracted by the activity of synthetic antibiofilm peptides. Overall, the results of this study demonstrate that factors within the bone environment and products of osteoblast-like cells directly influence S. aureus adhesion and could contribute to biofilm initiation on bone and/or prosthetics implants.
Keywords: antibiofilm peptides; bacterial starvation; biofilm; bone and joint infections; bone environment.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

