Inflammation-Nature's Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing "the Epidemic" of Chronic Diseases
- PMID: 30538987
- PMCID: PMC6277637
- DOI: 10.3389/fmed.2018.00316
Inflammation-Nature's Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing "the Epidemic" of Chronic Diseases
Abstract
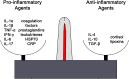
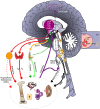
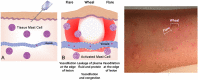
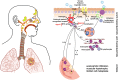
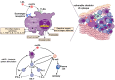
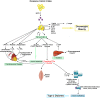
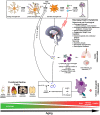
Siloed or singular system approach to disease management is common practice, developing out of traditional medical school education. Textbooks of medicine describe a huge number of discrete diseases, usually in a systematic fashion following headings like etiology, pathology, investigations, differential diagnoses, and management. This approach suggests that the body has a multitude of ways to respond to harmful incidences. However, physiology and systems biology provide evidence that there is a simple mechanism behind this phenotypical variability. Regardless if an injury or change was caused by trauma, infection, non-communicable disease, autoimmune disorders, or stress, the typical physiological response is: an increase in blood supply to the area, an increase in white cells into the affected tissue, an increase in phagocytic activity to remove the offending agent, followed by a down-regulation of these mechanisms resulting in healing. The cascade of inflammation is the body's unique mechanism to maintain its integrity in response to macroscopic as well as microscopic injuries. We hypothesize that chronic disease development and progression are linked to uncontrolled or dysfunctional inflammation to injuries regardless of their nature, physical, environmental, or psychological. Thus, we aim to reframe the prevailing approach of management of individual diseases into a more integrated systemic approach of treating the "person as a whole," enhancing the patient experience, ability to a make necessary changes, and maximize overall health and well-being. The first part of the paper reviews the local immune cascades of pro- and anti-inflammatory regulation and the interconnected feedback loops with neural and psychological pathways. The second part emphasizes one of nature's principles at work-system design and efficiency. Continually overwhelming this finely tuned system will result in systemic inflammation allowing chronic diseases to emerge; the pathways of several common conditions are described in detail. The final part of the paper considers the implications of these understandings for clinical care and explore how this lens could shape the physician-patient encounter and health system redesign. We conclude that healthcare professionals must advocate for an anti-inflammatory lifestyle at the patient level as well as at the local and national levels to enhance population health and well-being.
Keywords: anti-inflammatory lifestyle; chronic disease; clinical care; complex adaptive systems; inflammation; interdisciplinary; stress.
Figures





References
-
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. (1998) 105:83–107. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

