Domestic and International Shipping of Biospecimens
- PMID: 30539463
- PMCID: PMC6777724
- DOI: 10.1007/978-1-4939-8935-5_35
Domestic and International Shipping of Biospecimens
Abstract
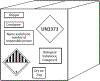
The packaging and shipment of biospecimens is a multistep process for which a distinct set of regulations needs to be followed, depending on whether a biospecimen is shipped domestically or internationally and whether the shipment contains hazardous materials. Shipments may be delayed if these regulations are not followed. Once learned, the process is straightforward. Major principles include double or triple packaging, adequate absorbent material, appropriate coolant, accurate labeling, and complete documentation. Training in packaging and shipping is often offered at major biomedical institutions and is a requirement to avoid shipping biohazards.
Keywords: Biobank; Biohazard; Biospecimen; Dry ice; Frozen; IATA; Shipping.
Figures
References
-
- American Society for Microbiology (2011) Packaging and Shipping Infectious Substances. http://dhmh.maryland.gov/laboratories/docs/ASM_Packing_and_Shipping_Infe.... Accessed 3 July 2015
-
- International Air Transit Association (2017) Dangerous Goods Regulations, 58th Editions. http://www.iata.org/whatwedo/cargo/dgr/Documents/infectious-substance-cl.... Accessed 20 Sept 2017
-
- AZoM (2006) CAS Numbers and UN Numbers – Identifications Systems for Materials and Chemical. https://www.azom.com/article.aspx?ArticleID=3506. Accessed 20 Sept 2017
-
- UN3373 Medical Packaging (2016) Regulations for UN3373. http://www.un3373.com/info/regulations/. Accessed 20 Sept 2017
-
- US Government Publishing Office (2011) Code of Federal Regulations. http://www.gpo.gov/fdsys/granule/CFR-2011-title49-vol2/CFR-2011-title49-.... Accessed 20 Aug 2015