Topography of 11C-Pittsburgh compound B uptake in Alzheimer's disease: a voxel-based investigation of cortical and white matter regions
- PMID: 30540022
- PMCID: PMC6781685
- DOI: 10.1590/1516-4446-2017-0002
Topography of 11C-Pittsburgh compound B uptake in Alzheimer's disease: a voxel-based investigation of cortical and white matter regions
Abstract
Objective: To compare results of positron emission tomography (PET) with carbon-11-labeled Pittsburgh compound B (11C-PIB) obtained with cerebellar or global brain uptake for voxel intensity normalization, describe the cortical sites with highest tracer uptake in subjects with mild Alzheimer's disease (AD), and explore possible group differences in 11C-PIB binding to white matter.
Methods: 11C-PIB PET scans were acquired from subjects with AD (n=17) and healthy elderly controls (n=19). Voxel-based analysis was performed with statistical parametric mapping (SPM).
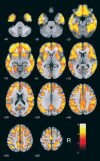
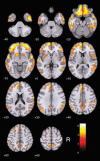
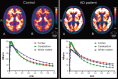
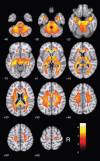
Results: Cerebellar normalization showed higher 11C-PIB uptake in the AD group relative to controls throughout the cerebral cortex, involving the lateral temporal, orbitofrontal, and superior parietal cortices. With global uptake normalization, greatest cortical binding was detected in the orbitofrontal cortex; decreased 11C-PIB uptake in white matter was found in the posterior hippocampal region, corpus callosum, pons, and internal capsule.
Conclusion: The present case-control voxelwise 11C-PIB PET comparison highlighted the regional distribution of amyloid deposition in the cerebral cortex of mildly demented AD patients. Tracer uptake was highest in the orbitofrontal cortex. Decreased 11C-PIB uptake in white-matter regions in this patient population may be a marker of white-matter damage in AD.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Toussaint PJ, Perlbarg V, Bellec P, Desarnaud S, Lacomblez L, Doyon J, et al. Resting state FDG-PET functional connectivity as an early biomarker of Alzheimer's disease using conjoint univariate and independent component analyses. Neuroimage. 2012;63:936–46. - PubMed
-
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound‐B. Ann Neurol. 2004;55:306–19. - PubMed
-
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47. - PubMed
-
- Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
