Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process
- PMID: 30540226
- PMCID: PMC6442924
- DOI: 10.1152/physrev.00005.2018
Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process
Abstract
The central functions fulfilled by mitochondria as both energy generators essential for tissue homeostasis and gateways to programmed apoptotic and necrotic cell death mandate tight control over the quality and quantity of these ubiquitous endosymbiotic organelles. Mitophagy, the targeted engulfment and destruction of mitochondria by the cellular autophagy apparatus, has conventionally been considered as the mechanism primarily responsible for mitochondrial quality control. However, our understanding of how, why, and under what specific conditions mitophagy is activated has grown tremendously over the past decade. Evidence is accumulating that nonmitophagic mitochondrial quality control mechanisms are more important to maintaining normal tissue homeostasis whereas mitophagy is an acute tissue stress response. Moreover, previously unrecognized mitophagic regulation of mitochondrial quantity control, metabolic reprogramming, and cell differentiation suggests that the mechanisms linking genetic or acquired defects in mitophagy to neurodegenerative and cardiovascular diseases or cancer are more complex than simple failure of normal mitochondrial quality control. Here, we provide a comprehensive overview of mitophagy in cellular homeostasis and disease and examine the most revolutionary concepts in these areas. In this context, we discuss evidence that atypical mitophagy and nonmitophagic pathways play central roles in mitochondrial quality control, functioning that was previously considered to be the primary domain of mitophagy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

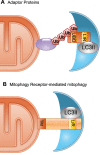
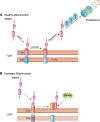
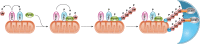
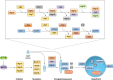
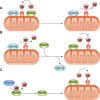
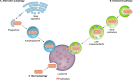
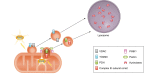
References
-
- Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, Götz A, Forsström S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, Suomalainen A. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab 15: 100–109, 2012. doi:10.1016/j.cmet.2011.11.012. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

