Coordinated regulation of transcription by CcpA and the Staphylococcus aureus two-component system HptRS
- PMID: 30540769
- PMCID: PMC6291074
- DOI: 10.1371/journal.pone.0207161
Coordinated regulation of transcription by CcpA and the Staphylococcus aureus two-component system HptRS
Abstract
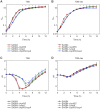
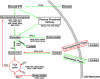
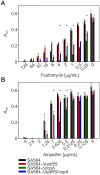
The success of Staphylococcus aureus as a pathogen is due in part to its ability to adapt to changing environmental conditions using signal transduction pathways, such as metabolite- responsive regulators and two-component systems. S. aureus has a two-component system encoded by the gene pair sav0224 (hptS) and sav0223 (hptR) that regulate the hexose phosphate transport (uhpT) system in response to extracellular glucose-6-phosphate. Glycolytic intermediates such as glucose-6-phosphate are important carbon sources that also modulate the activity of the global metabolite-responsive transcriptional regulator CcpA. Because uhpT has a putative CcpA binding site in its promoter and it is regulated by HptR, it was hypothesized the regulons of CcpA and HptR might intersect. To determine if the regulatory domains of CcpA and HptRS overlap, ccpA was deleted in strains SA564 and SA564-ΔhptRS and growth, metabolic, proteomic, and transcriptional differences were assessed. As expected, CcpA represses hptS and hptR in a glucose dependent manner; however, upon CcpA derepression, the HptRS system functions as a transcriptional activator of metabolic genes within the CcpA regulon. Importantly, inactivation of ccpA and hptRS altered sensitivity to fosfomycin and ampicillin in the absence of exogenous glucose-6-phosphate, indicating that both CcpA and HptRS modulate antibiotic susceptibility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, et al. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009;199: 1820–1826. 10.1086/599119 - DOI - PubMed
-
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5: 751–762. 10.1016/S1473-3099(05)70295-4 - DOI - PubMed
-
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13: 1840–1846. 10.3201/eid1312.070629 - DOI - PMC - PubMed
-
- Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nat Rev Microbiol. 2008;6: 657–666. 10.1038/nrmicro1955 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

