IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes
- PMID: 30540949
- PMCID: PMC6302550
- DOI: 10.1016/j.celrep.2018.11.064
IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes
Abstract
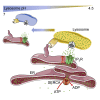
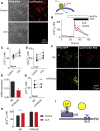
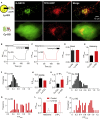
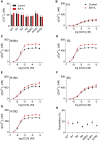
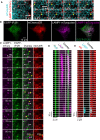
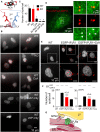
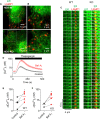
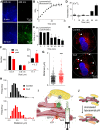
Inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) allow extracellular stimuli to redistribute Ca2+ from the ER to cytosol or other organelles. We show, using small interfering RNA (siRNA) and vacuolar H+-ATPase (V-ATPase) inhibitors, that lysosomes sequester Ca2+ released by all IP3R subtypes, but not Ca2+ entering cells through store-operated Ca2+ entry (SOCE). A low-affinity Ca2+ sensor targeted to lysosomal membranes reports large, local increases in cytosolic [Ca2+] during IP3-evoked Ca2+ release, but not during SOCE. Most lysosomes associate with endoplasmic reticulum (ER) and dwell at regions populated by IP3R clusters, but IP3Rs do not assemble ER-lysosome contacts. Increasing lysosomal pH does not immediately prevent Ca2+ uptake, but it causes lysosomes to slowly redistribute and enlarge, reduces their association with IP3Rs, and disrupts Ca2+ exchange with ER. In a "piston-like" fashion, ER concentrates cytosolic Ca2+ and delivers it, through large-conductance IP3Rs, to a low-affinity lysosomal uptake system. The involvement of IP3Rs allows extracellular stimuli to regulate Ca2+ exchange between the ER and lysosomes.
Keywords: Ca(2+); IP(3) receptor; concanamycin A; endoplasmic reticulum; genetically encoded Ca(2+) sensor; lysosome; membrane contact site; proximity ligation assay; store-operated Ca(2+) entry.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Getting close. Lysosome-ER contact sites tailor Ca2+ signals.Cell Calcium. 2019 Jun;80:194-196. doi: 10.1016/j.ceca.2019.02.003. Epub 2019 Feb 8. Cell Calcium. 2019. PMID: 30795844 No abstract available.
References
-
- Alpy F., Rousseau A., Schwab Y., Legueux F., Stoll I., Wendling C., Spiegelhalter C., Kessler P., Mathelin C., Rio M.C. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J. Cell Sci. 2013;126:5500–5512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

