Effect of Losartan and Fish Oil on Plasma IL-6 and Mobility in Older Persons. The ENRGISE Pilot Randomized Clinical Trial
- PMID: 30541065
- PMCID: PMC6748815
- DOI: 10.1093/gerona/gly277
Effect of Losartan and Fish Oil on Plasma IL-6 and Mobility in Older Persons. The ENRGISE Pilot Randomized Clinical Trial
Abstract
Background: Low-grade chronic inflammation, characterized by elevations in plasma Interleukin-6 (IL-6), is an independent risk factor of impaired mobility in older persons. Angiotensin receptor blockers and omega-3 polyunsaturated fatty acids (ω-3) may reduce IL-6 and may potentially improve physical function. To assess the main effects of the angiotensin receptor blocker losartan and ω-3 as fish oil on IL-6 and 400 m walking speed, we conducted the ENRGISE Pilot multicenter randomized clinical trial.
Methods: The ENRGISE Pilot enrolled participants between April 2016 and June 2017, who participated for 12 months. Participants were aged ≥70 years with mobility impairment, had IL-6 between 2.5 and 30 pg/mL, and were able to walk 400 m at baseline. Participants were randomized in three strata 2 × 2 factorial to: (i) losartan 50-100 mg/d or placebo (n = 43), (ii) fish oil 1,400-2,800 mg/d or placebo (n = 180), and (iii) with both (n = 66).
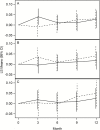
Results: Two hundred eighty-nine participants were randomized (mean age 78.3 years, 47.4% women, 17.0% black). There was no effect of losartan (difference of means = -0.065 ± 0.116 [SE], 95% confidence interval [CI]: -0.293-0.163, p = .58) or fish oil (-0.020 ± 0.077, 95% CI: -0.171-0.132, p = .80) on the log of IL-6. Similarly, there was no effect of losartan (-0.025 ± 0.026, 95% CI: -0.076-0.026, p = .34) or fish oil (0.010 ± 0.017, 95% CI: -0.025-0.044, p = .58) on walking speed (m/s).
Conclusions: These results do not support the use of these interventions to prevent mobility loss in older adults at risk of disability with low-grade chronic inflammation.
Registration: Clinicaltrials.gov NCT02676466.
Keywords: Aging; Factorial design; Inflammation; Multicenter trial; Walking speed.
© The Author(s) 2018. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Shumway-Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc. 2003;51:393–398. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

