Evidence that a catalytic glutamate and an 'Arginine Toggle' act in concert to mediate ATP hydrolysis and mechanochemical coupling in a viral DNA packaging motor
- PMID: 30541105
- PMCID: PMC6379665
- DOI: 10.1093/nar/gky1217
Evidence that a catalytic glutamate and an 'Arginine Toggle' act in concert to mediate ATP hydrolysis and mechanochemical coupling in a viral DNA packaging motor
Abstract
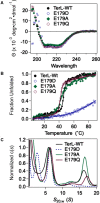
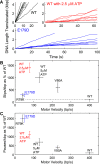
ASCE ATPases include ring-translocases such as cellular helicases and viral DNA packaging motors (terminases). These motors have conserved Walker A and B motifs that bind Mg2+-ATP and a catalytic carboxylate that activates water for hydrolysis. Here we demonstrate that Glu179 serves as the catalytic carboxylate in bacteriophage λ terminase and probe its mechanistic role. All changes of Glu179 are lethal: non-conservative changes abrogate ATP hydrolysis and DNA translocation, while the conservative E179D change attenuates ATP hydrolysis and alters single molecule translocation dynamics, consistent with a slowed chemical hydrolysis step. Molecular dynamics simulations of several homologous terminases suggest a novel mechanism, supported by experiments, wherein the conserved Walker A arginine 'toggles' between interacting with a glutamate residue in the 'lid' subdomain and the catalytic glutamate upon ATP binding; this switch helps mediate a transition from an 'open' state to a 'closed' state that tightly binds nucleotide and DNA, and also positions the catalytic glutamate next to the γ-phosphate to align the hydrolysis transition state. Concomitant reorientation of the lid subdomain may mediate mechanochemical coupling of ATP hydrolysis and DNA translocation. Given the strong conservation of these structural elements in terminase enzymes, this mechanism may be universal for viral packaging motors.
© The Author(s) 2018. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Walker-A Motif Acts to Coordinate ATP Hydrolysis with Motor Output in Viral DNA Packaging.J Mol Biol. 2016 Jul 3;428(13):2709-29. doi: 10.1016/j.jmb.2016.04.029. Epub 2016 Apr 30. J Mol Biol. 2016. PMID: 27139643 Free PMC article.
-
Defining the ATPase center of bacteriophage T4 DNA packaging machine: requirement for a catalytic glutamate residue in the large terminase protein gp17.J Mol Biol. 2003 Aug 1;331(1):139-54. doi: 10.1016/s0022-2836(03)00636-3. J Mol Biol. 2003. PMID: 12875841
-
Functional Dissection of a Viral DNA Packaging Machine's Walker B Motif.J Mol Biol. 2019 Nov 8;431(22):4455-4474. doi: 10.1016/j.jmb.2019.08.012. Epub 2019 Aug 30. J Mol Biol. 2019. PMID: 31473160 Free PMC article.
-
The bacteriophage DNA packaging motor.Annu Rev Genet. 2008;42:647-81. doi: 10.1146/annurev.genet.42.110807.091545. Annu Rev Genet. 2008. PMID: 18687036 Review.
-
The terminase enzyme from bacteriophage lambda: a DNA-packaging machine.Cell Mol Life Sci. 2000 Jan 20;57(1):128-48. doi: 10.1007/s000180050503. Cell Mol Life Sci. 2000. PMID: 10949585 Free PMC article. Review.
Cited by
-
Determining Trap Compliances, Microsphere Size Variations, and Response Linearities in Single DNA Molecule Elasticity Measurements with Optical Tweezers.Front Mol Biosci. 2021 Mar 22;8:605102. doi: 10.3389/fmolb.2021.605102. eCollection 2021. Front Mol Biosci. 2021. PMID: 33829038 Free PMC article.
-
ATP serves as a nucleotide switch coupling the genome maturation and packaging motor complexes of a virus assembly machine.Nucleic Acids Res. 2020 May 21;48(9):5006-5015. doi: 10.1093/nar/gkaa205. Nucleic Acids Res. 2020. PMID: 32255177 Free PMC article.
-
Controlling the Revolving and Rotating Motion Direction of Asymmetric Hexameric Nanomotor by Arginine Finger and Channel Chirality.ACS Nano. 2019 Jun 25;13(6):6207-6223. doi: 10.1021/acsnano.8b08849. Epub 2019 May 28. ACS Nano. 2019. PMID: 31067030 Free PMC article. Review.
-
Phenanthridine derivatives as potential HIV-1 protease inhibitors.Biomed Rep. 2020 Dec;13(6):66. doi: 10.3892/br.2020.1373. Epub 2020 Oct 20. Biomed Rep. 2020. PMID: 33149910 Free PMC article.
-
Viral packaging ATPases utilize a glutamate switch to couple ATPase activity and DNA translocation.Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2024928118. doi: 10.1073/pnas.2024928118. Proc Natl Acad Sci U S A. 2021. PMID: 33888587 Free PMC article.
References
-
- Vale R.D. The molecular motor toolbox for intracellular transport. Cell. 2003; 112:467–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

