Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions
- PMID: 30541144
- PMCID: PMC6424003
- DOI: 10.1210/er.2018-00154
Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions
Abstract
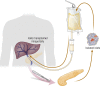
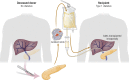
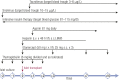
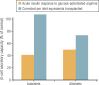
Pancreatic islet transplantation has become an established approach to β-cell replacement therapy for the treatment of insulin-deficient diabetes. Recent progress in techniques for islet isolation, islet culture, and peritransplant management of the islet transplant recipient has resulted in substantial improvements in metabolic and safety outcomes for patients. For patients requiring total or subtotal pancreatectomy for benign disease of the pancreas, isolation of islets from the diseased pancreas with intrahepatic transplantation of autologous islets can prevent or ameliorate postsurgical diabetes, and for patients previously experiencing painful recurrent acute or chronic pancreatitis, quality of life is substantially improved. For patients with type 1 diabetes or insulin-deficient forms of pancreatogenic (type 3c) diabetes, isolation of islets from a deceased donor pancreas with intrahepatic transplantation of allogeneic islets can ameliorate problematic hypoglycemia, stabilize glycemic lability, and maintain on-target glycemic control, consequently with improved quality of life, and often without the requirement for insulin therapy. Because the metabolic benefits are dependent on the numbers of islets transplanted that survive engraftment, recipients of autoislets are limited to receive the number of islets isolated from their own pancreas, whereas recipients of alloislets may receive islets isolated from more than one donor pancreas. The development of alternative sources of islet cells for transplantation, whether from autologous, allogeneic, or xenogeneic tissues, is an active area of investigation that promises to expand access and indications for islet transplantation in the future treatment of diabetes.
Copyright © 2019 Endocrine Society.
Figures

References
-
- Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. - PubMed
-
- Reckard CR, Barker CF. Transplantation of isolated pancreatic-islets across strong and weak histocompatibility barriers. Transplant Proc. 1973;5:761–763. - PubMed
-
- Barker CF, Reckard CR, Ziegler MM, Naji A. Liver as an immunologically privileged site for rat pancreatic-islet allografts. Diabetes. 1975;24:418.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

