Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin
- PMID: 30541350
- PMCID: PMC6430695
- DOI: 10.1080/02656736.2018.1550815
Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin
Abstract
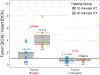
Thermosensitive liposomal doxorubicin (LTSL-Dox) combined with mild hyperthermia enhances the localized delivery of doxorubicin (Dox) within a heated region. The optimal heating duration and the impact of extended heating on systemic drug distribution are unknown. Here we evaluated local and systemic Dox delivery with two different mild hyperthermia durations (42 °C for 10 or 40 minutes) in a Vx2 rabbit tumor model. We hypothesized that longer duration of hyperthermia would increase Dox concentration in heated tumors without increasing systemic exposure. Temporally and spatially accurate controlled hyperthermia was achieved using a clinical MR-HIFU system for the prescribed heating durations. Forty-minutes of heating resulted in a nearly 6-fold increase in doxorubicin concentration in heated vs unheated tumors in the same animals. Therapeutic ratio, defined as the ratio of Dox delivered into the heated tumor vs the heart, increased from 1.9-fold with 10 minutes heating to 4.4-fold with 40 minutes heating. MR-HIFU can be used to guide, deliver and monitor mild hyperthermia of a Vx2 tumor model in a rabbit model, and an increased duration of heating leads to higher Dox deposition from LTSL-Dox in a target tumor without a concomitant increase in systemic exposure. Results from this preclinical study can be used to help establish clinical treatment protocols for hyperthermia mediated drug delivery.
Keywords: MR-guided high intensity focused ultrasound; Targeted drug delivery; mild hyperthermia; therapeutic ratio; thermosensitive liposomal doxorubicin.
Figures
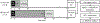

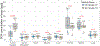
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
