Insights into the transcriptional and post-transcriptional regulation of the rice SUMOylation machinery and into the role of two rice SUMO proteases
- PMID: 30541427
- PMCID: PMC6291987
- DOI: 10.1186/s12870-018-1547-3
Insights into the transcriptional and post-transcriptional regulation of the rice SUMOylation machinery and into the role of two rice SUMO proteases
Abstract
Background: SUMOylation is an essential eukaryotic post-translation modification that, in plants, regulates numerous cellular processes, ranging from seed development to stress response. Using rice as a model crop plant, we searched for potential regulatory points that may influence the activity of the rice SUMOylation machinery genes.
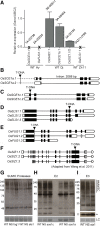
Results: We analyzed the presence of putative cis-acting regulatory elements (CREs) within the promoter regions of the rice SUMOylation machinery genes and found CREs related to different cellular processes, including hormone signaling. We confirmed that the transcript levels of genes involved in target-SUMOylation, containing ABA- and GA-related CREs, are responsive to treatments with these hormones. Transcriptional analysis in Nipponbare (spp. japonica) and LC-93-4 (spp. indica), showed that the transcript levels of all studied genes are maintained in the two subspecies, under normal growth. OsSUMO3 is an exceptional case since it is expressed at low levels or is not detectable at all in LC-93-4 roots and shoots, respectively. We revealed post-transcriptional regulation by alternative splicing (AS) for all genes studied, except for SUMO coding genes, OsSIZ2, OsOTS3, and OsELS2. Some AS forms have the potential to alter protein domains and catalytic centers. We also performed the molecular and phenotypic characterization of T-DNA insertion lines of some of the genes under study. Knockouts of OsFUG1 and OsELS1 showed increased SUMOylation levels and non-overlapping phenotypes. The fug1 line showed a dwarf phenotype, and significant defects in fertility, seed weight, and panicle architecture, while the els1 line showed early flowering and decreased plant height. We suggest that OsELS1 is an ortholog of AtEsd4, which was also supported by our phylogenetic analysis.
Conclusions: Overall, we provide a comprehensive analysis of the rice SUMOylation machinery and discuss possible effects of the regulation of these genes at the transcriptional and post-transcriptional level. We also contribute to the characterization of two rice SUMO proteases, OsELS1 and OsFUG1.
Keywords: Alternative splicing; Rice (Oryza sativa); SUMO proteases; SUMOylation; T-DNA; cis-elements.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Functional characterization of the SIZ/PIAS-type SUMO E3 ligases, OsSIZ1 and OsSIZ2 in rice.Plant Cell Environ. 2010 Nov;33(11):1923-34. doi: 10.1111/j.1365-3040.2010.02195.x. Plant Cell Environ. 2010. PMID: 20561251
-
Rice SUMO protease Overly Tolerant to Salt 1 targets the transcription factor, OsbZIP23 to promote drought tolerance in rice.Plant J. 2017 Dec;92(6):1031-1043. doi: 10.1111/tpj.13739. Epub 2017 Nov 17. Plant J. 2017. PMID: 29024118
-
Characterization of novel SUMO family genes in the rice genome.Genes Genet Syst. 2021 May 8;96(1):25-32. doi: 10.1266/ggs.20-00034. Epub 2021 Mar 17. Genes Genet Syst. 2021. PMID: 33731501
-
Rice SUMOs and unification of their names.Genes Genet Syst. 2023 Jun 23;98(1):1-7. doi: 10.1266/ggs.22-00097. Epub 2023 May 2. Genes Genet Syst. 2023. PMID: 37150617 Review.
-
Fifty shades of SUMO: its role in immunity and at the fulcrum of the growth-defence balance.Mol Plant Pathol. 2018 Jun;19(6):1537-1544. doi: 10.1111/mpp.12625. Epub 2018 Feb 13. Mol Plant Pathol. 2018. PMID: 29024335 Free PMC article. Review.
Cited by
-
Stress-Induced Changes in Alternative Splicing Landscape in Rice: Functional Significance of Splice Isoforms in Stress Tolerance.Biology (Basel). 2021 Apr 8;10(4):309. doi: 10.3390/biology10040309. Biology (Basel). 2021. PMID: 33917813 Free PMC article. Review.
-
Molecular Characterization and Functional Localization of a Novel SUMOylation Gene in Oryza sativa.Biology (Basel). 2021 Dec 31;11(1):53. doi: 10.3390/biology11010053. Biology (Basel). 2021. PMID: 35053052 Free PMC article.
-
Genome-wide in silico analysis of long intergenic non-coding RNAs from rice peduncles at the heading stage.Physiol Mol Biol Plants. 2021 Oct;27(10):2389-2406. doi: 10.1007/s12298-021-01059-2. Epub 2021 Oct 12. Physiol Mol Biol Plants. 2021. PMID: 34744373 Free PMC article.
-
High temperature-mediated disturbance of carbohydrate metabolism and gene expressional regulation in rice: a review.Plant Signal Behav. 2021 Mar 4;16(3):1862564. doi: 10.1080/15592324.2020.1862564. Epub 2021 Jan 20. Plant Signal Behav. 2021. PMID: 33470154 Free PMC article. Review.
-
Dealing With Stress: A Review of Plant SUMO Proteases.Front Plant Sci. 2019 Sep 18;10:1122. doi: 10.3389/fpls.2019.01122. eCollection 2019. Front Plant Sci. 2019. PMID: 31620153 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

