Cell salvage using the continuous autotransfusion device CATSmart - an observational bicenter technical evaluation
- PMID: 30541447
- PMCID: PMC6292025
- DOI: 10.1186/s12871-018-0651-0
Cell salvage using the continuous autotransfusion device CATSmart - an observational bicenter technical evaluation
Abstract
Background: The use of cell salvage and autologous blood transfusion has become an important method of blood conservation. So far, there are no clinical data about the performance of the continuous autotransfusion device CATSmart.
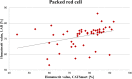
Methods: In total, 74 patients undergoing either cardiac or orthopedic surgery were included in this prospective, bicenter and observational technical evaluation to validate red cell separation process and washout quality of CATSmart. The target of red cell separation process was defined as a hematocrit value in the packed red cell unit of 55-75% and of washout quality of 80-100% removal ratio.
Results: Hematocrit values measured by CATSmart and laboratory analysis were 78.5% [71.3%; 84.0%] and 73.7% [67.5%; 75.5%], respectively. Removal ratios for platelets 94.7% [88.2%; 96.7%], free hemoglobin 89.3% [85.2%; 94.9%], albumin 97.9% [96.6%; 98.5%], heparin 99.9% [99.9%; 100.0%], and potassium 92.5% [90.8%; 95.0%] were within the target range while removal of white blood cells was slightly worse 72.4% [57.9%; 87.3%].
Conclusion: The new autotransfusion device enables sufficient red cell separation and washout quality.
Keywords: Auto transfusion; Cell salvage; Hematocrit value.
Conflict of interest statement
Ethics approval and consent to participate
The ethics committee of the University Hospital Frankfurt approved the protocol addressing patients undergoing cardiac surgery (approval ref. 80/15). A written consent of the participations was not required according to national regulations (German Constitution Art. 72, Berufsordnung für Ärzte §15, HeilBerG Nordrhein-Westfalen §7). This technical evaluation was not required and thus not submitted to IRB of the Marienhospital Bottrop (Ärztekammer Westfalen Lippe) according to regional and national regulations (German Constitution Art. 72, Berufsordnung für Ärzte §15, HeilBerG Nordrhein-Westfalen §7).
Consent for publication
Not applicable.
Competing interests
PM and KZ received grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt‘s Patient Blood Management program and honoraria for scientific lectures from B. Braun Melsungen, Vifor Pharma, Fearing, CSL Behring, and Pharmacosmos.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Fresenius Kabi. CATSmart - https://www.fresenius-kabi.com/in/products/catsmart. 22.10.2018.
-
- Bundesärztekammer. Richtlinie zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Richtlinie Hämotherapie);17.02.2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

