Discrepancies in endpoints between clinical trial protocols and clinical trial registration in randomized trials in oncology
- PMID: 30541475
- PMCID: PMC6292048
- DOI: 10.1186/s12874-018-0627-2
Discrepancies in endpoints between clinical trial protocols and clinical trial registration in randomized trials in oncology
Abstract
Background: Clinical trials are an essential part of evidence-based medicine. Hence, to ensure transparency and accountability in these clinical trials, policies for registration have been framed with emphasis on mandatory submission of trial elements, specifically outcome measures. As these efforts evolve further, we sought to evaluate the current status of endpoint reporting in clinical trial registries.
Methods: We reviewed 71 oncology related randomized controlled trials published in three high impact journals. We compared primary (PEP) and non-primary endpoints (NPEP) between the clinical trial protocols of these trials and their corresponding registration in one of the 14 primary global clinical trial registries. A discrepancy was defined as the non-reporting or absence of an endpoint in either the protocol or registry. The primary endpoint was the rate of discrepancy between secondary endpoints in clinical trial protocols and clinical trial registries.
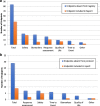
Results: Of the 71 clinical trials, a discrepancy in PEP was found in only 4 trials (6%). Secondary endpoint (SEP) differences were found in 45 (63%) trials. Among these 45 trials, 36 (80%) had SEPs that were planned in the protocol but not reported in the registry and 19 (42%) had SEPs with endpoints in the registry that were not found in the protocol. The total number of SEPs that were absent from the corresponding registry and protocol were 84 and 29, respectively. Of these endpoints, 48 (57%) and 9 (31%) were included in the published report of these trials.
Conclusion: Although recent regulations and enhanced procedures have improved the number and quality of clinical trial registrations, inconsistencies regarding endpoint reporting still exist. Though further guidelines for the registration of clinical trials will help, greater efforts to provide a correct, easily accessible, and complete representation of planned endpoints are needed.
Keywords: Endpoints; Registry; Reporting.
Conflict of interest statement
Ethics approval and consent to participate
This study was exempt from institutional review board approval because we used only publicly available data and did not include any human subjects or patient health data.
Consent for publication
There were no human subjects or patient health data used.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Comparison of Registered and Reported Outcomes in Randomized Clinical Trials Published in Anesthesiology Journals.Anesth Analg. 2017 Oct;125(4):1292-1300. doi: 10.1213/ANE.0000000000002272. Anesth Analg. 2017. PMID: 28704247
-
Registration status and outcome reporting of trials published in core headache medicine journals.Neurology. 2015 Nov 17;85(20):1789-94. doi: 10.1212/WNL.0000000000002127. Epub 2015 Oct 16. Neurology. 2015. PMID: 26475691 Review.
-
Quality of Reporting in Oncology Randomized Controlled Trials: From 2011 to 2015.Cancer Control. 2018 Jan-Mar;25(1):1073274818781309. doi: 10.1177/1073274818781309. Cancer Control. 2018. PMID: 29895186 Free PMC article.
-
Discrepancies Between Randomized Controlled Trial Registry Entries and Content of Corresponding Manuscripts Reported in Anesthesiology Journals.Anesth Analg. 2015 Oct;121(4):1030-1033. doi: 10.1213/ANE.0000000000000824. Anesth Analg. 2015. PMID: 26378702
-
Comparison between publicly accessible publications, registries, and protocols of phase III trials indicated persistence of selective outcome reporting.J Clin Epidemiol. 2017 Nov;91:87-94. doi: 10.1016/j.jclinepi.2017.07.010. Epub 2017 Jul 27. J Clin Epidemiol. 2017. PMID: 28757260 Review.
Cited by
-
Unveiling the Promise: Navigating Clinical Trials 1978-2024 for PDAC.Cancers (Basel). 2024 Oct 23;16(21):3564. doi: 10.3390/cancers16213564. Cancers (Basel). 2024. PMID: 39518005 Free PMC article. Review.
-
An analysis of controlled human infection studies registered on ClinicalTrials.gov.BMJ Open. 2025 Feb 7;15(2):e085250. doi: 10.1136/bmjopen-2024-085250. BMJ Open. 2025. PMID: 39920069 Free PMC article.
-
Incidence of Primary End Point Changes Among Active Cancer Phase 3 Randomized Clinical Trials.JAMA Netw Open. 2023 May 1;6(5):e2313819. doi: 10.1001/jamanetworkopen.2023.13819. JAMA Netw Open. 2023. PMID: 37195664 Free PMC article. Clinical Trial.
-
Trends of clinical trials from 2017 to 2019 in Korea: an integrated analysis based on the Ministry of Food and Drug Safety (MFDS) and the Clinical Research Information Service (CRIS) registries.Transl Clin Pharmacol. 2021 Dec;29(4):186-196. doi: 10.12793/tcp.2021.29.e24. Epub 2021 Dec 21. Transl Clin Pharmacol. 2021. PMID: 35024359 Free PMC article.
-
Secondary Endpoint Utilization and Publication Rate among Phase III Oncology Trials.Cancer Res Commun. 2024 Aug 1;4(8):2183-2188. doi: 10.1158/2767-9764.CRC-24-0265. Cancer Res Commun. 2024. PMID: 39099199 Free PMC article.
References
-
- Public Law 105–115. Food and Drug Administration Modernization Act of 1997 (FDAMA). 1997;111 STAT. 2296.
-
- Clinicaltrials.gov. (2017. ClinicalTrials.gov Background- ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ct2/about-site/background (Accessed 1 Oct 2017].
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous