Association of high pressure and alkaline condition for solubilization of inclusion bodies and refolding of the NS1 protein from zika virus
- PMID: 30541520
- PMCID: PMC6291932
- DOI: 10.1186/s12896-018-0486-2
Association of high pressure and alkaline condition for solubilization of inclusion bodies and refolding of the NS1 protein from zika virus
Abstract
Background: Proteins in inclusion bodies (IBs) present native-like secondary structures. However, chaotropic agents at denaturing concentrations, which are widely used for IB solubilization and subsequent refolding, unfold these secondary structures. Removal of the chaotropes frequently causes reaggregation and poor recovery of bioactive proteins. High hydrostatic pressure (HHP) and alkaline pH are two conditions that, in the presence of low level of chaotropes, have been described as non-denaturing solubilization agents. In the present study we evaluated the strategy of combination of HHP and alkaline pH on the solubilization of IB using as a model an antigenic form of the zika virus (ZIKV) non-structural 1 (NS1) protein.
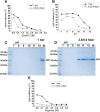
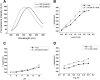
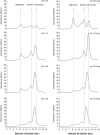
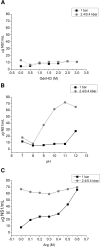
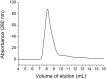
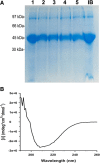
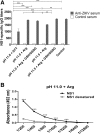
Results: Pressure-treatment (2.4 kbar) of NS1-IBs at a pH of 11.0 induced a low degree of NS1 unfolding and led to solubilization of the IBs, mainly into monomers. After dialysis at pH 8.5, NS1 was refolded and formed soluble oligomers. High (up to 68 mg/liter) NS1 concentrations were obtained by solubilization of NS1-IBs at pH 11 in the presence of arginine (Arg) with a final yield of approximately 80% of total protein content. The process proved to be efficient, quick and did not require further purification steps. Refolded NS1 preserved biological features regarding reactivity with antigen-specific antibodies, including sera of ZIKV-infected patients. The method resulted in an increase of approximately 30-fold over conventional IB solubilization-refolding methods.
Conclusions: The present results represent an innovative non-denaturing protein refolding process by means of the concomitant use of HHP and alkaline pH. Application of the reported method allowed the recovery of ZIKV NS1 at a condition that maintained the antigenic properties of the protein.
Keywords: Alkaline pH; Dengue virus and Zika virus; High hydrostatic pressure; Inclusion bodies; NS1; Protein refolding.
Conflict of interest statement
Ethics approval and consent to participate
The Institute of Biosciences of the University of São Paulo review board approved this study and all human sera were collected under the approval of the Ethics Committee (CEPSH - Off.011616).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Rathore AS, Bade P, Joshi V, Pathak M, Pattanayek SK. Refolding of biotech therapeutic proteins expressed in bacteria: review. J Chem Technol Biotechnol. 2013;8810:1794–1806. doi: 10.1002/jctb.4152. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

