Hypoxia and connectivity in the developing vertebrate nervous system
- PMID: 30541748
- PMCID: PMC6307895
- DOI: 10.1242/dmm.037127
Hypoxia and connectivity in the developing vertebrate nervous system
Abstract
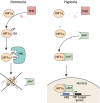
The developing nervous system depends upon precise regulation of oxygen levels. Hypoxia, the condition of low oxygen concentration, can interrupt developmental sequences and cause a range of molecular, cellular and neuronal changes and injuries. The roles and effects of hypoxia on the central nervous system (CNS) are poorly characterized, even though hypoxia is simultaneously a normal component of development, a potentially abnormal environmental stressor in some settings, and a clinically important complication, for example of prematurity. Work over the past decade has revealed that hypoxia causes specific disruptions in the development of CNS connectivity, altering axon pathfinding and synapse development. The goals of this article are to review hypoxia's effects on the development of CNS connectivity, including its genetic and molecular mediators, and the changes it causes in CNS circuitry and function due to regulated as well as unintended mechanisms. The transcription factor HIF1α is the central mediator of the CNS response to hypoxia (as it is elsewhere in the body), but hypoxia also causes a dysregulation of gene expression. Animals appear to have evolved genetic and molecular responses to hypoxia that result in functional behavioral alterations to adapt to the changes in oxygen concentration during CNS development. Understanding the molecular pathways underlying both the normal and abnormal effects of hypoxia on CNS connectivity may reveal novel insights into common neurodevelopmental disorders. In addition, this Review explores the current gaps in knowledge, and suggests important areas for future studies.
Keywords: Connectivity; Hypoxia; Neuroscience; Pathfinding.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Bartoszewska S., Kochan K., Piotrowski A., Kamysz W., Ochocka R. J., Collawn J. F. and Bartoszewski R. (2015). The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1alpha expression in human endothelial cells through a negative feedback loop. FASEB J. 29, 1467-1479. 10.1096/fj.14-267054 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

