Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding
- PMID: 30541778
- PMCID: PMC6291622
- DOI: 10.1128/mSphere.00564-18
Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding
Abstract
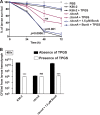
Burkholderia cenocepacia is an opportunistic Gram-negative bacterium that causes serious respiratory infections in patients with cystic fibrosis. Recently, we discovered that B. cenocepacia produces the extracellular bacterial lipocalin protein BcnA upon exposure to sublethal concentrations of bactericidal antibiotics. BcnA captures a range of antibiotics outside bacterial cells, providing a global extracellular mechanism of antimicrobial resistance. In this study, we investigated water-soluble and liposoluble forms of vitamin E as inhibitors of antibiotic binding by BcnA. Our results demonstrate that in vitro, both vitamin E forms bind strongly to BcnA and contribute to reduce the MICs of norfloxacin (a fluoroquinolone) and ceftazidime (a β-lactam), both of them used as model molecules representing two different chemical classes of antibiotics. Expression of BcnA was required for the adjuvant effect of vitamin E. These results were replicated in vivo using the Galleria mellonella larva infection model whereby vitamin E treatment, in combination with norfloxacin, significantly increased larva survival upon infection in a BcnA-dependent manner. Together, our data suggest that vitamin E can be used to increase killing by bactericidal antibiotics through interference with lipocalin binding.IMPORTANCE Bacteria exposed to stress mediated by sublethal antibiotic concentrations respond by adaptive mechanisms leading to an overall increase of antibiotic resistance. One of these mechanisms involves the release of bacterial proteins called lipocalins, which have the ability to sequester antibiotics in the extracellular space before they reach bacterial cells. We speculated that interfering with lipocalin-mediated antibiotic binding could enhance the efficacy of antibiotics to kill bacteria. In this work, we report that when combined with bactericidal antibiotics, vitamin E contributes to enhance bacterial killing both in vitro and in vivo. This adjuvant effect of vitamin E requires the presence of BcnA, a bacterial lipocalin produced by the cystic fibrosis pathogen Burkholderia cenocepacia Since most bacteria produce lipocalins like BcnA, we propose that our findings could be translated into making novel antibiotic adjuvants to potentiate bacterial killing by existing antibiotics.
Keywords: Gram-negative bacteria; antibiotic resistance; chronic infection; cystic fibrosis; intrinsic resistance; lipocalin; vitamin E.
Copyright © 2018 Naguib and Valvano.
Figures






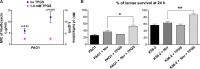
Similar articles
-
Antibiotic Capture by Bacterial Lipocalins Uncovers an Extracellular Mechanism of Intrinsic Antibiotic Resistance.mBio. 2017 Mar 14;8(2):e00225-17. doi: 10.1128/mBio.00225-17. mBio. 2017. PMID: 28292982 Free PMC article.
-
Overcoming an Extremely Drug Resistant (XDR) Pathogen: Avibactam Restores Susceptibility to Ceftazidime for Burkholderia cepacia Complex Isolates from Cystic Fibrosis Patients.ACS Infect Dis. 2017 Jul 14;3(7):502-511. doi: 10.1021/acsinfecdis.7b00020. Epub 2017 Mar 30. ACS Infect Dis. 2017. PMID: 28264560 Free PMC article.
-
The antibacterial properties of docosahexaenoic omega-3 fatty acid against the cystic fibrosis multiresistant pathogen Burkholderia cenocepacia.FEMS Microbiol Lett. 2012 Mar;328(1):61-9. doi: 10.1111/j.1574-6968.2011.02476.x. Epub 2012 Jan 6. FEMS Microbiol Lett. 2012. PMID: 22150831
-
Molecular approaches to pathogenesis study of Burkholderia cenocepacia, an important cystic fibrosis opportunistic bacterium.Appl Microbiol Biotechnol. 2011 Dec;92(5):887-95. doi: 10.1007/s00253-011-3616-5. Epub 2011 Oct 14. Appl Microbiol Biotechnol. 2011. PMID: 21997606 Review.
-
Vaccines to Overcome Antibiotic Resistance: The Challenge of Burkholderia cenocepacia.Trends Microbiol. 2020 Apr;28(4):315-326. doi: 10.1016/j.tim.2019.12.005. Epub 2020 Jan 10. Trends Microbiol. 2020. PMID: 31932141 Review.
Cited by
-
Evaluation of Antimicrobial and Anticancer Activities of Bouea macrophylla Ethanol Extract.Methods Mol Biol. 2022;2343:215-228. doi: 10.1007/978-1-0716-1558-4_14. Methods Mol Biol. 2022. PMID: 34473325
-
Effect of Castor and Cashew Nut Shell Oils, Selenium and Vitamin E as Antioxidants on the Health and Meat Stability of Lambs Fed a High-Concentrate Diet.Antioxidants (Basel). 2020 Dec 18;9(12):1298. doi: 10.3390/antiox9121298. Antioxidants (Basel). 2020. PMID: 33353112 Free PMC article.
-
Potential Antimicrobial and Anticancer Activities of an Ethanol Extract from Bouea macrophylla.Molecules. 2020 Apr 24;25(8):1996. doi: 10.3390/molecules25081996. Molecules. 2020. PMID: 32344601 Free PMC article.
-
GC-MS Analysis, Antibacterial and Antioxidant Potential of Ethyl Acetate Leaf Extract of Senna singueana (Delile) Grown in Kenya.Evid Based Complement Alternat Med. 2022 Aug 18;2022:5436476. doi: 10.1155/2022/5436476. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36034966 Free PMC article.
-
Vitamin E for Prevention of Biofilm-caused Healthcare-associated Infections.Open Med (Wars). 2019 Dec 26;15:14-21. doi: 10.1515/med-2020-0004. eCollection 2018. Open Med (Wars). 2019. PMID: 31922015 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

