Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain
- PMID: 30541786
- PMCID: PMC8170511
- DOI: 10.1126/scitranslmed.aan0237
Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain
Abstract
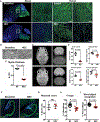
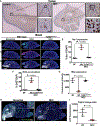
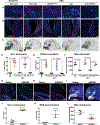
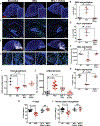
Necrotizing enterocolitis (NEC) is a severe gastrointestinal disease of the premature infant. One of the most important long-term complications observed in children who survive NEC early in life is the development of profound neurological impairments. However, the pathways leading to NEC-associated neurological impairments remain unknown, thus limiting the development of prevention strategies. We have recently shown that NEC development is dependent on the expression of the lipopolysaccharide receptor Toll-like receptor 4 (TLR4) on the intestinal epithelium, whose activation by bacteria in the newborn gut leads to mucosal inflammation. Here, we hypothesized that damage-induced production of TLR4 endogenous ligands in the intestine might lead to activation of microglial cells in the brain and promote cognitive impairments. We identified a gut-brain signaling axis in an NEC mouse model in which activation of intestinal TLR4 signaling led to release of high-mobility group box 1 in the intestine that, in turn, promoted microglial activation in the brain and neurological dysfunction. We further demonstrated that an orally administered dendrimer-based nanotherapeutic approach to targeting activated microglia could prevent NEC-associated neurological dysfunction in neonatal mice. These findings shed light on the molecular pathways leading to the development of NEC-associated brain injury, provide a rationale for early removal of diseased intestine in NEC, and indicate the potential of targeted therapies that protect the developing brain in the treatment of NEC in early childhood.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
Comment in
-
Deciphering the gut-brain link in NEC.Nat Rev Immunol. 2019 Feb;19(2):70-71. doi: 10.1038/s41577-018-0115-2. Nat Rev Immunol. 2019. PMID: 30604774 No abstract available.
-
Preventing brain damage in necrotizing enterocolitis.Nat Rev Gastroenterol Hepatol. 2019 Feb;16(2):75. doi: 10.1038/s41575-019-0107-0. Nat Rev Gastroenterol Hepatol. 2019. PMID: 30643226 No abstract available.
Similar articles
-
Glycyrrhizin alleviates brain injury in necrotizing enterocolitis model mice by suppressing HMGB1/TLR4 pathway.Int Immunopharmacol. 2025 Mar 26;150:114294. doi: 10.1016/j.intimp.2025.114294. Epub 2025 Feb 18. Int Immunopharmacol. 2025. PMID: 39970710
-
Pulmonary Epithelial TLR4 Activation Leads to Lung Injury in Neonatal Necrotizing Enterocolitis.J Immunol. 2016 Aug 1;197(3):859-71. doi: 10.4049/jimmunol.1600618. Epub 2016 Jun 15. J Immunol. 2016. PMID: 27307558 Free PMC article.
-
The human milk oligosaccharides 2'-fucosyllactose and 6'-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling.Pediatr Res. 2021 Jan;89(1):91-101. doi: 10.1038/s41390-020-0852-3. Epub 2020 Mar 27. Pediatr Res. 2021. PMID: 32221473 Free PMC article.
-
Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis.Cell Mol Gastroenterol Hepatol. 2018 Apr 6;6(2):229-238.e1. doi: 10.1016/j.jcmgh.2018.04.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30105286 Free PMC article. Review.
-
New insights into the pathogenesis of necrotizing enterocolitis and the dawn of potential therapeutics.Semin Pediatr Surg. 2023 Jun;32(3):151309. doi: 10.1016/j.sempedsurg.2023.151309. Epub 2023 Jun 1. Semin Pediatr Surg. 2023. PMID: 37290338 Free PMC article. Review.
Cited by
-
Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates.Pediatr Res. 2022 Apr;91(5):1182-1195. doi: 10.1038/s41390-021-01614-3. Epub 2021 Jun 8. Pediatr Res. 2022. PMID: 34103675 Free PMC article.
-
Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice.J Neuroinflammation. 2021 Mar 6;18(1):66. doi: 10.1186/s12974-021-02111-4. J Neuroinflammation. 2021. PMID: 33676524 Free PMC article.
-
Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury.Dis Model Mech. 2019 Oct 21;12(10):dmm040808. doi: 10.1242/dmm.040808. Dis Model Mech. 2019. PMID: 31537532 Free PMC article.
-
Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain.J Neuroinflammation. 2019 May 10;16(1):97. doi: 10.1186/s12974-019-1481-9. J Neuroinflammation. 2019. PMID: 31077225 Free PMC article.
-
Prenatal Immunity and Influences on Necrotizing Enterocolitis and Associated Neonatal Disorders.Front Immunol. 2021 Apr 21;12:650709. doi: 10.3389/fimmu.2021.650709. eCollection 2021. Front Immunol. 2021. PMID: 33968047 Free PMC article. Review.
References
-
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015). - PMC - PubMed
-
- Hamilton BE, Martin JA, Osterman MJ, Births: Preliminary data for 2015. Natl. Vital Stat. Rep. 65, 1–15 (2016). - PubMed
-
- Leaphart CL, Cavallo JC, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ, A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

