Verdinexor (KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro
- PMID: 30541831
- PMCID: PMC6364025
- DOI: 10.1128/JVI.01684-18
Verdinexor (KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro
Abstract
Respiratory syncytial virus (RSV) is a leading cause of hospitalization of infants and young children, causing considerable respiratory disease and repeat infections that may lead to chronic respiratory conditions such as asthma, wheezing, and bronchitis. RSV causes ∼34 million new episodes of lower respiratory tract illness (LRTI) in children younger than 5 years of age, with >3 million hospitalizations due to severe RSV-associated LRTI. The standard of care is limited to symptomatic relief as there are no approved vaccines and few effective antiviral drugs; thus, a safe and efficacious RSV therapeutic is needed. Therapeutic targeting of host proteins hijacked by RSV to facilitate replication is a promising antiviral strategy as targeting the host reduces the likelihood of developing drug resistance. The nuclear export of the RSV M protein, mediated by the nuclear export protein exportin 1 (XPO1), is crucial for RSV assembly and budding. Inhibition of RSV M protein export by leptomycin B correlated with reduced RSV replication in vitro In this study, we evaluated the anti-RSV efficacy of Verdinexor (KPT-335), a small molecule designed to reversibly inhibit XPO1-mediated nuclear export. KPT-335 inhibited XPO1-mediated transport and reduced RSV replication in vitro KPT-335 was effective against RSV A and B strains and reduced viral replication following prophylactic or therapeutic administration. Inhibition of RSV replication by KPT-335 was due to a combined effect of reduced XPO1 expression, disruption of the nuclear export of RSV M protein, and inactivation of the NF-κB signaling pathway.IMPORTANCE RSV is an important cause of LRTI in infants and young children for which there are no suitable antiviral drugs offered. We evaluated the efficacy of KPT-335 as an anti-RSV drug and show that KPT-335 inhibits XPO1-mediated nuclear export, leading to nuclear accumulation of RSV M protein and reduction in RSV levels. KPT-335 treatment also resulted in inhibition of proinflammatory pathways, which has important implications for its effectiveness in vivo.
Keywords: KPT-335; M protein; RSV; Verdinexor; XPO1; exportin 1; nuclear export; respiratory syncytial virus.
Copyright © 2019 American Society for Microbiology.
Figures




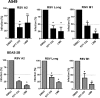
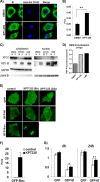
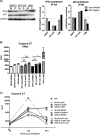

Similar articles
-
Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo.J Virol. 2014 Sep 1;88(17):10228-43. doi: 10.1128/JVI.01774-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965445 Free PMC article.
-
Verdinexor Targeting of CRM1 is a Promising Therapeutic Approach against RSV and Influenza Viruses.Viruses. 2018 Jan 21;10(1):48. doi: 10.3390/v10010048. Viruses. 2018. PMID: 29361733 Free PMC article. Review.
-
Reversible disruption of XPO1-mediated nuclear export inhibits respiratory syncytial virus (RSV) replication.Sci Rep. 2021 Sep 28;11(1):19223. doi: 10.1038/s41598-021-98767-2. Sci Rep. 2021. PMID: 34584169 Free PMC article.
-
KPT-330 inhibitor of XPO1-mediated nuclear export has anti-proliferative activity in hepatocellular carcinoma.Cancer Chemother Pharmacol. 2014 Sep;74(3):487-95. doi: 10.1007/s00280-014-2495-8. Epub 2014 Jul 17. Cancer Chemother Pharmacol. 2014. PMID: 25030088 Free PMC article.
-
Nucleo-cytoplasmic transport as a therapeutic target of cancer.J Hematol Oncol. 2014 Dec 5;7:85. doi: 10.1186/s13045-014-0085-1. J Hematol Oncol. 2014. PMID: 25476752 Free PMC article. Review.
Cited by
-
COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics.Infection. 2021 Apr;49(2):199-213. doi: 10.1007/s15010-020-01516-2. Epub 2020 Sep 4. Infection. 2021. PMID: 32886331 Free PMC article.
-
Drug Combinations as a First Line of Defense against Coronaviruses and Other Emerging Viruses.mBio. 2021 Dec 21;12(6):e0334721. doi: 10.1128/mbio.03347-21. Epub 2021 Dec 21. mBio. 2021. PMID: 34933447 Free PMC article. Review.
-
Immunopathology of RSV: An Updated Review.Viruses. 2021 Dec 10;13(12):2478. doi: 10.3390/v13122478. Viruses. 2021. PMID: 34960746 Free PMC article. Review.
-
Selinexor and COVID-19: The Neglected Warden.Front Pharmacol. 2022 Apr 26;13:884228. doi: 10.3389/fphar.2022.884228. eCollection 2022. Front Pharmacol. 2022. PMID: 35559257 Free PMC article. Review.
-
Screening Drugs for Broad-Spectrum, Host-Directed Antiviral Activity: Lessons from the Development of Probenecid for COVID-19.Viruses. 2023 Nov 14;15(11):2254. doi: 10.3390/v15112254. Viruses. 2023. PMID: 38005930 Free PMC article.
References
-
- Iwane MK, Edwards KM, Szilagyi PG, Walker FJ, Griffin MR, Weinberg GA, Coulen C, Poehling KA, Shone LP, Balter S, Hall CB, Erdman DD, Wooten K, Schwartz B, New Vaccine SN. 2004. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 113:1758–1764. doi:10.1542/peds.113.6.1758. - DOI - PubMed
-
- Falsey AR, Walsh EE, Looney RJ, Kolassa JE, Formica MA, Criddle MC, Hall WJ. 1999. Comparison of respiratory syncytial virus humoral immunity and response to infection in young and elderly adults. J Med Virol 59:221–226. doi:10.1002/(SICI)1096-9071(199910)59:2<221::AID-JMV16>3.0.CO;2-H. - DOI - PubMed
-
- Collins PL. 2011. Human respiratory syncytial virus, p 341–410. In Samal SK. (ed), The biology of paramyxoviruses. Caister Academic Press, Norfolk, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

