Global Proteomic Profiling of Salmonella Infection by a Giant Phage
- PMID: 30541839
- PMCID: PMC6384053
- DOI: 10.1128/JVI.01833-18
Global Proteomic Profiling of Salmonella Infection by a Giant Phage
Abstract
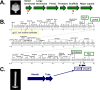
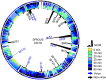
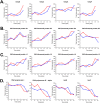
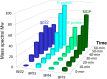
The 240-kb Salmonella phage SPN3US genome encodes 264 gene products, many of which are functionally uncharacterized. We have previously used mass spectrometry to define the proteomes of wild-type and mutant forms of the SPN3US virion. In this study, we sought to determine whether this technique was suitable for the characterization of the SPN3US proteome during liquid infection. Mass spectrometry of SPN3US-infected cells identified 232 SPN3US and 1,994 Salmonella proteins. SPN3US proteins with related functions, such as proteins with roles in DNA replication, transcription, and virion formation, were coordinately expressed in a temporal manner. Mass spectral counts showed the four most abundant SPN3US proteins to be the major capsid protein, two head ejection proteins, and the functionally unassigned protein gp22. This high abundance of gp22 in infected bacteria contrasted with its absence from mature virions, suggesting that it might be the scaffold protein, an essential head morphogenesis protein yet to be identified in giant phages. We identified homologs to SPN3US gp22 in 45 related giant phages, including ϕKZ, whose counterpart is also abundant in infected bacteria but absent in the virion. We determined the ϕKZ counterpart to be cleaved in vitro by its prohead protease, an event that has been observed to promote head maturation of some other phages. Our findings are consistent with a scaffold protein assignment for SPN3US gp22, although direct evidence is required for its confirmation. These studies demonstrate the power of mass spectral analyses for facilitating the acquisition of new knowledge into the molecular events of viral infection.IMPORTANCE "Giant" phages with genomes >200 kb are being isolated in increasing numbers from a range of environments. With hosts such as Salmonella enterica, Pseudomonas aeruginosa, and Erwinia amylovora, these phages are of interest for phage therapy of multidrug-resistant pathogens. However, our understanding of how these complex phages interact with their hosts is impeded by the proportion (∼80%) of their gene products that are functionally uncharacterized. To develop the repertoire of techniques for analysis of phages, we analyzed a liquid infection of Salmonella phage SPN3US (240-kb genome) using third-generation mass spectrometry. We observed the temporal production of phage proteins whose genes collectively represent 96% of the SPN3US genome. These findings demonstrate the sensitivity of mass spectrometry for global proteomic profiling of virus-infected cells, and the identification of a candidate for a major head morphogenesis protein will facilitate further studies into giant phage head assembly.
Keywords: Salmonella; bacteriophage assembly; giant phage; mass spectrometry.
Figures

Similar articles
-
A Cut above the Rest: Characterization of the Assembly of a Large Viral Icosahedral Capsid.Viruses. 2020 Jul 5;12(7):725. doi: 10.3390/v12070725. Viruses. 2020. PMID: 32635654 Free PMC article.
-
Identification of Essential Genes in the Salmonella Phage SPN3US Reveals Novel Insights into Giant Phage Head Structure and Assembly.J Virol. 2016 Oct 28;90(22):10284-10298. doi: 10.1128/JVI.01492-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27605673 Free PMC article.
-
To Be or Not To Be T4: Evidence of a Complex Evolutionary Pathway of Head Structure and Assembly in Giant Salmonella Virus SPN3US.Front Microbiol. 2017 Nov 15;8:2251. doi: 10.3389/fmicb.2017.02251. eCollection 2017. Front Microbiol. 2017. PMID: 29187846 Free PMC article.
-
Structure and physiology of giant DNA viruses.Curr Opin Virol. 2021 Aug;49:58-67. doi: 10.1016/j.coviro.2021.04.012. Epub 2021 May 26. Curr Opin Virol. 2021. PMID: 34051592 Review.
-
Use of high-throughput mass spectrometry to elucidate host-pathogen interactions in Salmonella.Future Microbiol. 2008 Dec;3(6):625-34. doi: 10.2217/17460913.3.6.625. Future Microbiol. 2008. PMID: 19072180 Free PMC article. Review.
Cited by
-
The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell.Viruses. 2020 Aug 19;12(9):910. doi: 10.3390/v12090910. Viruses. 2020. PMID: 32825132 Free PMC article.
-
A Cut above the Rest: Characterization of the Assembly of a Large Viral Icosahedral Capsid.Viruses. 2020 Jul 5;12(7):725. doi: 10.3390/v12070725. Viruses. 2020. PMID: 32635654 Free PMC article.
-
Multi-interface licensing of protein import into a phage nucleus.Nature. 2025 Mar;639(8054):456-462. doi: 10.1038/s41586-024-08547-x. Epub 2025 Feb 5. Nature. 2025. PMID: 39910297 Free PMC article.
-
A cytoskeletal vortex drives phage nucleus rotation during jumbo phage replication in E. coli.Cell Rep. 2022 Aug 16;40(7):111179. doi: 10.1016/j.celrep.2022.111179. Cell Rep. 2022. PMID: 35977483 Free PMC article.
-
Characterization and complete genome sequence of Privateer, a highly prolate Proteus mirabilis podophage.PeerJ. 2021 Feb 10;9:e10645. doi: 10.7717/peerj.10645. eCollection 2021. PeerJ. 2021. PMID: 33614267 Free PMC article.
References
-
- Hendrix RW. 2009. Jumbo bacteriophages, p 229–240. In Van Etten J. (ed), Lesser known large dsDNA viruses, vol 328 Springer, Berlin, Germany. - PubMed
-
- Serwer P, Hayes SJ, Thomas J, Demeler B, Hardies SC. 2009. Isolation of novel large and aggregating bacteriophages, p 55–66. In Clokie M, Kropinski AM (ed), Bacteriophages: methods and protocols. Humana Press, New York, NY. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

