Role of Sphingomyelin in Alphaherpesvirus Entry
- PMID: 30541840
- PMCID: PMC6384054
- DOI: 10.1128/JVI.01547-18
Role of Sphingomyelin in Alphaherpesvirus Entry
Abstract
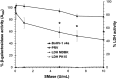
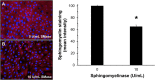
Bovine herpesvirus 1 (BoHV-1) is an alphaherpesvirus that causes disease in cattle populations worldwide. Sphingomyelin (SM) is the most abundant sphingolipid in the mammalian cell membrane, where it preferentially associates with cholesterol to form lipid raft domains. SM is a substrate for the lysosome-resident enzyme acid sphingomyelinase, which plays a role in cell membrane repair following injury. Treatment of cells with noncytotoxic concentrations of Staphylococcus aureus-derived sphingomyelinase successfully reduced cell surface-exposed sphingomyelin but did not significantly inhibit BoHV-1 entry and infection, as measured by the beta-galactosidase reporter assay. Interestingly, entry of the porcine alphaherpesvirus pseudorabies virus (PRV) was inhibited by sphingomyelin-depletion of cells. Treatment of BoHV-1 particles with sphingomyelinase inhibited viral entry activity, suggesting that viral SM plays a role in BoHV-1 entry, while cellular SM does not. Treatment of cells with noncytotoxic concentrations of the functional inhibitors of host acid sphingomyelinase, imipramine and amitriptyline, which induce degradation of the cellular enzyme, did not significantly inhibit BoHV-1 entry. In contrast, inhibition of cellular acid sphingomyelinase inhibited PRV entry. Entry of the human alphaherpesvirus herpes simplex virus 1 (HSV-1) was independent of both host SM and acid sphingomyelinase, in a manner similar to BoHV-1. Together, the results suggest that among the alphaherpesviruses, there is variability in entry requirements for cellular sphingomyelin and acid sphingomyelinase activity.IMPORTANCE Bovine herpesvirus 1 (BoHV-1) is an ubiquitous pathogen affecting cattle populations worldwide. Infection can result in complicated, polymicrobial infections due to the immunosuppressive properties of the virus. Available vaccines limit disease severity and spread but do not prevent infection. The financial and animal welfare ramifications of BoHV-1 are significant. In order to develop more effective prevention and treatment regimens, a more complete understanding of the initial steps in viral infection is necessary. We recently identified a low pH endocytosis pathway for BoHV-1. Here, we examine the role of cellular factors responsible for membrane integrity and repair in alphaherpesviral entry. This study allows comparisons of the BoHV-1 entry pathway with those of other alphaherpesviruses (pseudorabies virus [PRV] and herpes simplex virus 1 [HSV-1]). Lastly, this is the first report of sphingomyelin and lysosomal sphingomyelinase playing a role in the entry of a herpesvirus. The results may lead to the development of more effective prevention and treatment regimens.
Keywords: bovine herpesvirus 1; endocytosis; herpes simplex virus; herpesviruses; membranes; pseudorabies virus; sphingomyelin; viral entry.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Bovine Herpesvirus 1 Entry by a Low-pH Endosomal Pathway.J Virol. 2018 Sep 26;92(20):e00839-18. doi: 10.1128/JVI.00839-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045989 Free PMC article.
-
Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus.J Virol. 2019 Jan 4;93(2):e01849-18. doi: 10.1128/JVI.01849-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355685 Free PMC article.
-
The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier.mBio. 2024 Nov 13;15(11):e0187324. doi: 10.1128/mbio.01873-24. Epub 2024 Oct 9. mBio. 2024. PMID: 39382295 Free PMC article.
-
Distinctive features of bovine alphaherpesvirus types 1 and 5 and the virus-host interactions that might influence clinical outcomes.Arch Virol. 2020 Feb;165(2):285-301. doi: 10.1007/s00705-019-04494-5. Epub 2019 Dec 16. Arch Virol. 2020. PMID: 31845150 Review.
-
Biology of bovine herpesvirus 5.Vet J. 2010 May;184(2):138-45. doi: 10.1016/j.tvjl.2009.03.035. Epub 2009 May 5. Vet J. 2010. PMID: 19409823 Review.
Cited by
-
Membrane Sphingomyelin in Host Cells Is Essential for Nucleocapsid Penetration into the Cytoplasm after Hemifusion during Rubella Virus Entry.mBio. 2022 Dec 20;13(6):e0169822. doi: 10.1128/mbio.01698-22. Epub 2022 Nov 8. mBio. 2022. PMID: 36346228 Free PMC article.
-
Host cellular factors involved in pseudorabies virus attachment and entry: a mini review.Front Vet Sci. 2023 Nov 27;10:1314624. doi: 10.3389/fvets.2023.1314624. eCollection 2023. Front Vet Sci. 2023. PMID: 38089700 Free PMC article. Review.
-
HIV and SIV Envelope Glycoproteins Interact with Glycolipids and Lipids.Int J Mol Sci. 2023 Jul 21;24(14):11730. doi: 10.3390/ijms241411730. Int J Mol Sci. 2023. PMID: 37511488 Free PMC article.
-
Sphingolipids: Effectors and Achilles Heals in Viral Infections?Cells. 2021 Aug 24;10(9):2175. doi: 10.3390/cells10092175. Cells. 2021. PMID: 34571822 Free PMC article. Review.
-
A Genome-Wide CRISPR/Cas9 Screen Reveals the Requirement of Host Sphingomyelin Synthase 1 for Infection with Pseudorabies Virus Mutant gD-Pass.Viruses. 2021 Aug 9;13(8):1574. doi: 10.3390/v13081574. Viruses. 2021. PMID: 34452438 Free PMC article.
References
-
- Leon FC, Diez FG, Ferri FR, Vizcaino LL, Gijon FC, Gimeno EJ, Sein CZ, Rodriguez JMSV, Madrigal JJC, Gomez PC, Schudel A. 2005. The translation into Spanish of the OIE Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees): problems, solutions and conclusions. Rev Sci Tech 24:1095–1104. - PubMed
-
- Constable P, Hinchcliff KW, Done S, Gruenberg W. 2017. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats and horses, 11th ed Saunders Ltd; Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

