Coadministration of CH31 Broadly Neutralizing Antibody Does Not Affect Development of Vaccine-Induced Anti-HIV-1 Envelope Antibody Responses in Infant Rhesus Macaques
- PMID: 30541851
- PMCID: PMC6384077
- DOI: 10.1128/JVI.01783-18
Coadministration of CH31 Broadly Neutralizing Antibody Does Not Affect Development of Vaccine-Induced Anti-HIV-1 Envelope Antibody Responses in Infant Rhesus Macaques
Abstract
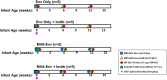
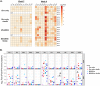
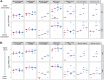
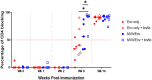
Prevention of mother-to-child transmission (MTCT) is an indispensable component in combatting the global AIDS epidemic. A combination of passive broadly neutralizing antibody (bnAb) infusion and active vaccination promises to provide protection of infants against MTCT from birth through the breastfeeding period and could prime the immune system for lifelong immunity. In this study, we investigate the impact of a single infusion of CD4 binding site (CD4bs) bnAb administered at birth on de novo antibody responses elicited by concurrent active HIV envelope vaccination. Four groups of infant macaques received active immunizations with subunit Env protein or modified vaccinia Ankara (MVA)-vectored Env and subunit Env protein, with or without a single intravenous coadministration of CH31 bnAb at birth. Vaccinated animals were monitored to evaluate binding and functional antibody responses elicited by the active vaccinations. Despite achieving plasma concentrations that were able to neutralize tier 2 viruses, coadministration of CH31 did not have a large impact on the kinetics, magnitude, specificity, or avidity of vaccine-elicited binding or functional antibody responses, including epitope specificity, the development of CD4bs antibodies, neutralization, binding to infected cells, or antibody-dependent cell-mediated cytotoxicity (ADCC). We conclude that infusion of CD4bs bnAb CH31 at birth does not interfere with de novo antibody responses to active vaccination and that a combination of passive bnAb infusion and active HIV-1 Env vaccination is a viable strategy for immediate and prolonged protection against MTCT.IMPORTANCE Our study is the first to evaluate the impact of passive infusion of a broadly neutralizing antibody in newborns on the de novo development of antibody responses following active vaccinations in infancy. We demonstrated the safety and the feasibility of bnAb administration to achieve biologically relevant levels of the antibody and showed that the passive infusion did not impair the de novo antibody production following HIV-1 Env vaccination. Our study paves the way for further investigations of the combination strategy using passive plus active immunization to provide protection of infants born to HIV-1-positive mothers over the entire period of risk for mother-to-child transmission.
Keywords: AIDS vaccine; HIV; antibody; broadly neutralizing antibody; infant; nonhuman primate; passive immunization; pediatric vaccine.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Impact of Poxvirus Vector Priming, Protein Coadministration, and Vaccine Intervals on HIV gp120 Vaccine-Elicited Antibody Magnitude and Function in Infant Macaques.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00231-17. doi: 10.1128/CVI.00231-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28814388 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Combined HIV-1 Envelope Systemic and Mucosal Immunization of Lactating Rhesus Monkeys Induces a Robust Immunoglobulin A Isotype B Cell Response in Breast Milk.J Virol. 2016 Apr 29;90(10):4951-4965. doi: 10.1128/JVI.00335-16. Print 2016 May 15. J Virol. 2016. PMID: 26937027 Free PMC article.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Progress in HIV vaccine development.Hum Vaccin Immunother. 2017 May 4;13(5):1018-1030. doi: 10.1080/21645515.2016.1276138. Epub 2017 Mar 10. Hum Vaccin Immunother. 2017. PMID: 28281871 Free PMC article. Review.
Cited by
-
AIDS Vaccine Research Subcommittee (AVRS) Consultation: Early-Life Immunization Strategies against HIV Acquisition.mSphere. 2019 Jul 17;4(4):e00320-19. doi: 10.1128/mSphere.00320-19. mSphere. 2019. PMID: 31315966 Free PMC article.
-
HIV Env-Specific IgG Antibodies Induced by Vaccination of Neonatal Rhesus Macaques Persist and Can Be Augmented by a Late Booster Immunization in Infancy.mSphere. 2020 Mar 25;5(2):e00162-20. doi: 10.1128/mSphere.00162-20. mSphere. 2020. PMID: 32213623 Free PMC article.
-
Early Post-Vaccination Gene Signatures Correlate With the Magnitude and Function of Vaccine-Induced HIV Envelope-Specific Plasma Antibodies in Infant Rhesus Macaques.Front Immunol. 2022 Apr 27;13:840976. doi: 10.3389/fimmu.2022.840976. eCollection 2022. Front Immunol. 2022. PMID: 35572573 Free PMC article.
-
Dam-Infant Rhesus Macaque Pairs to Dissect Age-Dependent Responses to SARS-CoV-2 Infection.Immunohorizons. 2022 Dec 1;6(12):851-863. doi: 10.4049/immunohorizons.2200075. Immunohorizons. 2022. PMID: 36547390 Free PMC article.
-
Vaccine-Induced, High-Magnitude HIV Env-Specific Antibodies with Fc-Mediated Effector Functions Are Insufficient to Protect Infant Rhesus Macaques against Oral SHIV Infection.mSphere. 2022 Feb 23;7(1):e0083921. doi: 10.1128/msphere.00839-21. Epub 2022 Feb 23. mSphere. 2022. PMID: 35196125 Free PMC article.
References
-
- UNAIDS. 2018. Global HIV & AIDS statistics—2018 fact sheet. UNAIDS, Geneva, Switzerland. http://www.unaids.org/en/resources/fact-sheet.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

