HIV Infection Functionally Impairs Mycobacterium tuberculosis-Specific CD4 and CD8 T-Cell Responses
- PMID: 30541853
- PMCID: PMC6384080
- DOI: 10.1128/JVI.01728-18
HIV Infection Functionally Impairs Mycobacterium tuberculosis-Specific CD4 and CD8 T-Cell Responses
Abstract
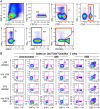
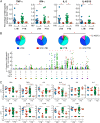
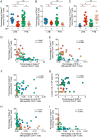
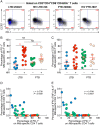
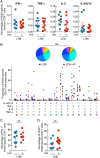
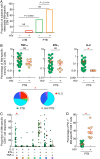
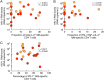
Human immunodeficiency virus (HIV) infection is the major risk factor predisposing for Mycobacterium tuberculosis progression from latent tuberculosis infection (LTBI) to tuberculosis disease (TB). Since long-term-treated aviremic HIV-infected individuals remained at higher risk of developing TB than HIV-uninfected individuals, we hypothesized that progression from LTBI to pulmonary TB (PTB) might be due not only to CD4 T-cell depletion but also to M. tuberculosis-specific CD4 T-cell functional impairment. To test this hypothesis, M. tuberculosis-specific T-cell frequencies and cytokine profiles were investigated in untreated Tanzanian individuals suffering from LTBI (n = 20) or PTB (n = 67) and compared to those of untreated M. tuberculosis/HIV-coinfected individuals suffering from LTBI (n = 15) or PTB (n = 10). We showed that HIV infection significantly reduced the proportion of Th2 (interleukin 4 [IL-4]/IL-5/IL-13) producing M. tuberculosis-specific CD4 T cells and IL-2-producing M. tuberculosis-specific CD4 and CD8 T cells in individuals with LTBI or PTB (P < 0.05). Interestingly, the loss of IL-2 production was associated with a significant increase of PD-1 expression on M. tuberculosis-specific CD4 and CD8 T cells (P < 0.05), while the loss of Th2 cytokine production was associated with a significant reduction of Gata-3 expression in memory CD4 T cells (P < 0.05). Finally, we showed that the serum levels of IL-1α, IL-6, C-reactive protein (CRP), IL-23, and IP-10 were significantly reduced in M. tuberculosis/HIV-coinfected individuals with PTB compared to those in HIV-negative individuals with PTB (P < 0.05), suggesting that HIV infection significantly suppresses M. tuberculosis-induced systemic proinflammatory cytokine responses. Taken together, this study suggests that in addition to depleting M. tuberculosis-specific CD4 T cells, HIV infection significantly impairs functionally favorable M. tuberculosis-specific CD4 T-cell responses in Tanzanian individuals with LTBI or PTB.IMPORTANCEMycobacterium tuberculosis and human immunodeficiency virus (HIV) infections are coendemic in several regions of the world, and M. tuberculosis/HIV-coinfected individuals are more susceptible to progression to tuberculosis disease. We therefore hypothesized that HIV infection would potentially impair M. tuberculosis-specific protective immunity in individuals suffering from latent tuberculosis infection (LTBI) or active pulmonary tuberculosis (PTB). In this study, we demonstrated that M. tuberculosis/HIV-coinfected individuals have fewer circulating M. tuberculosis-specific CD4 T cells and that those that remained were functionally impaired in both LTBI and PTB settings. In addition, we showed that HIV infection significantly interferes with M. tuberculosis-induced systemic proinflammatory cytokine/chemokine responses. Taken together, these data suggest that HIV infection impairs functionally favorable M. tuberculosis-specific immunity.
Keywords: CD4 T cells; HIV; Mycobacterium tuberculosis; exhaustion.
Copyright © 2019 Amelio et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

