Splicing-Dependent Subcellular Targeting of Borna Disease Virus Nucleoprotein Isoforms
- PMID: 30541858
- PMCID: PMC6384089
- DOI: 10.1128/JVI.01621-18
Splicing-Dependent Subcellular Targeting of Borna Disease Virus Nucleoprotein Isoforms
Abstract
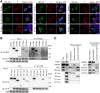
Targeting of viral proteins to specific subcellular compartments is a fundamental step for viruses to achieve successful replication in infected cells. Borna disease virus 1 (BoDV-1), a nonsegmented, negative-strand RNA virus, uniquely replicates and persists in the cell nucleus. Here, it is demonstrated that BoDV nucleoprotein (N) transcripts undergo mRNA splicing to generate truncated isoforms. In combination with alternative usage of translation initiation sites, the N gene potentially expresses at least six different isoforms, which exhibit diverse intracellular localizations, including the nucleoplasm, cytoplasm, and endoplasmic reticulum (ER), as well as intranuclear viral replication sites. Interestingly, the ER-targeting signal peptide in N is exposed by removing the intron by mRNA splicing. Furthermore, the spliced isoforms inhibit viral polymerase activity. Consistently, recombinant BoDVs lacking the N-splicing signals acquire the ability to replicate faster than wild-type virus in cultured cells, suggesting that N isoforms created by mRNA splicing negatively regulate BoDV replication. These results provided not only the mechanism of how mRNA splicing generates viral proteins that have distinct functions but also a novel strategy for replication control of RNA viruses using isoforms with different subcellular localizations.IMPORTANCE Borna disease virus (BoDV) is a highly neurotropic RNA virus that belongs to the orthobornavirus genus. A zoonotic orthobornavirus that is genetically related to BoDV has recently been identified in squirrels, thus increasing the importance of understanding the replication and pathogenesis of orthobornaviruses. BoDV replicates in the nucleus and uses alternative mRNA splicing to express viral proteins. However, it is unknown whether the virus uses splicing to create protein isoforms with different functions. The present study demonstrated that the nucleoprotein transcript undergoes splicing and produces four new isoforms in coordination with alternative usage of translation initiation codons. The spliced isoforms showed a distinct intracellular localization, including in the endoplasmic reticulum, and recombinant viruses lacking the splicing signals replicated more efficiently than the wild type. The results provided not only a new regulation of BoDV replication but also insights into how RNA viruses produce protein isoforms from small genomes.
Keywords: Mononegavirales; RNA splicing; bornavirus.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
The Borna Disease Virus 2 (BoDV-2) Nucleoprotein Is a Conspecific Protein That Enhances BoDV-1 RNA-Dependent RNA Polymerase Activity.J Virol. 2021 Oct 13;95(21):e0093621. doi: 10.1128/JVI.00936-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406860 Free PMC article.
-
Generation of a non-transmissive Borna disease virus vector lacking both matrix and glycoprotein genes.Microbiol Immunol. 2017 Sep;61(9):380-386. doi: 10.1111/1348-0421.12505. Microbiol Immunol. 2017. PMID: 28776750
-
Dual function of the nuclear export signal of the Borna disease virus nucleoprotein in nuclear export activity and binding to viral phosphoprotein.Virol J. 2017 Jul 11;14(1):126. doi: 10.1186/s12985-017-0793-6. Virol J. 2017. PMID: 28693611 Free PMC article.
-
Reverse genetics approaches of Borna disease virus: applications in development of viral vectors and preventive vaccines.Curr Opin Virol. 2020 Oct;44:42-48. doi: 10.1016/j.coviro.2020.05.011. Epub 2020 Jul 10. Curr Opin Virol. 2020. PMID: 32659515 Review.
-
The atypical strategies used for gene expression of Borna disease virus, a nonsegmented, negative-strand RNA virus.Uirusu. 1995 Dec;45(2):165-74. doi: 10.2222/jsv.45.165. Uirusu. 1995. PMID: 8820535 Review.
Cited by
-
Optimal Expression of the Envelope Glycoprotein of Orthobornaviruses Determines the Production of Mature Virus Particles.J Virol. 2021 Mar 1;95(5):e02221-20. doi: 10.1128/JVI.02221-20. Epub 2020 Dec 2. J Virol. 2021. PMID: 33268525 Free PMC article.
-
The Borna Disease Virus 2 (BoDV-2) Nucleoprotein Is a Conspecific Protein That Enhances BoDV-1 RNA-Dependent RNA Polymerase Activity.J Virol. 2021 Oct 13;95(21):e0093621. doi: 10.1128/JVI.00936-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406860 Free PMC article.
-
Reverse genetics of parrot bornavirus 4 reveals a unique splicing of the glycoprotein gene that affects viral propagation.J Virol. 2023 Aug 31;97(8):e0050923. doi: 10.1128/jvi.00509-23. Epub 2023 Aug 14. J Virol. 2023. PMID: 37578232 Free PMC article.
-
The hidden diversity of ancient bornaviral sequences from X and P genes in vertebrate genomes.Virus Evol. 2023 Jun 3;9(1):vead038. doi: 10.1093/ve/vead038. eCollection 2023. Virus Evol. 2023. PMID: 37360682 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

