A Highly Attenuated Vesicular Stomatitis Virus-Based Vaccine Platform Controls Hepatitis B Virus Replication in Mouse Models of Hepatitis B
- PMID: 30541859
- PMCID: PMC6384079
- DOI: 10.1128/JVI.01586-18
A Highly Attenuated Vesicular Stomatitis Virus-Based Vaccine Platform Controls Hepatitis B Virus Replication in Mouse Models of Hepatitis B
Abstract
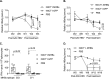
Therapeutic vaccines may be an important component of a treatment regimen for curing chronic hepatitis B virus (HBV) infection. We previously demonstrated that recombinant wild-type vesicular stomatitis virus (VSV) expressing the HBV middle surface glycoprotein (MHBs) elicits functional immune responses in mouse models of HBV replication. However, VSV has some undesirable pathogenic properties, and the use of this platform in humans requires further viral attenuation. We therefore generated a highly attenuated VSV that expresses MHBs and contains two attenuating mutations. This vector was evaluated for immunogenicity, pathogenesis, and anti-HBV function in mice. Compared to wild-type VSV, the highly attenuated virus displayed markedly reduced pathogenesis but induced similar MHBs-specific CD8+ T cell and antibody responses. The CD8+ T cell responses elicited by this vector in naive mice prevented HBV replication in animals that were later challenged by hydrodynamic injection or transduction with adeno-associated virus encoding the HBV genome (AAV-HBV). In mice in which persistent HBV replication was first established by AAV-HBV transduction, subsequent immunization with the attenuated VSV induced MHBs-specific CD8+ T cell responses that corresponded with reductions in serum and liver HBV antigens and nucleic acids. HBV control was associated with an increase in the frequency of intrahepatic HBV-specific CD8+ T cells and a transient elevation in serum alanine aminotransferase activity. The ability of VSV to induce a robust multispecific T cell response that controls HBV replication combined with the improved safety profile of the highly attenuated vector suggests that this platform offers a new approach for HBV therapeutic vaccination.IMPORTANCE A curative treatment for chronic hepatitis B must eliminate the virus from the liver, but current antiviral therapies typically fail to do so. Immune-mediated resolution of infection occurs in a small fraction of chronic HBV patients, which suggests the potential efficacy of therapeutic strategies that boost the patient's own immune response to the virus. We modified a safe form of VSV to express an immunogenic HBV protein and evaluated the efficacy of this vector in the prevention and treatment of HBV infection in mouse models. Our results show that this vector elicits HBV-specific immune responses that prevent the establishment of HBV infection and reduce viral proteins in the serum and viral DNA/RNA in the liver of mice with persistent HBV replication. These findings suggest that highly attenuated and safe virus-based vaccine platforms have the potential to be utilized for the development of an effective therapeutic vaccine against chronic HBV infection.
Keywords: adeno-associated virus; chronic hepatitis B; immunotherapy; immunotolerance; therapeutic vaccines; vesicular stomatitis virus.
Copyright © 2019 American Society for Microbiology.
Figures







Similar articles
-
Advances in Targeting the Innate and Adaptive Immune Systems to Cure Chronic Hepatitis B Virus Infection.Front Immunol. 2020 Feb 7;10:3127. doi: 10.3389/fimmu.2019.03127. eCollection 2019. Front Immunol. 2020. PMID: 32117201 Free PMC article. Review.
-
Heterologous prime-boost immunization with vesiculovirus-based vectors expressing HBV Core antigen induces CD8+ T cell responses in naïve and persistently infected mice and protects from challenge.Antiviral Res. 2019 Aug;168:156-167. doi: 10.1016/j.antiviral.2019.05.014. Epub 2019 May 30. Antiviral Res. 2019. PMID: 31153968 Free PMC article.
-
Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection.J Virol. 2015 Oct;89(20):10407-15. doi: 10.1128/JVI.01184-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246574 Free PMC article.
-
A vesicular stomatitis virus-based therapeutic vaccine generates a functional CD8 T cell response to hepatitis B virus in transgenic mice.J Virol. 2013 Mar;87(5):2969-73. doi: 10.1128/JVI.02111-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269785 Free PMC article.
-
Therapeutic vaccination against chronic hepatitis B virus infection.J Clin Virol. 2005 Dec;34 Suppl 1:S108-14. doi: 10.1016/s1386-6532(05)80019-8. J Clin Virol. 2005. PMID: 16461209 Review.
Cited by
-
Advances in Targeting the Innate and Adaptive Immune Systems to Cure Chronic Hepatitis B Virus Infection.Front Immunol. 2020 Feb 7;10:3127. doi: 10.3389/fimmu.2019.03127. eCollection 2019. Front Immunol. 2020. PMID: 32117201 Free PMC article. Review.
-
Virus-based vaccine vectors with distinct replication mechanisms differentially infect and activate dendritic cells.NPJ Vaccines. 2021 Nov 22;6(1):138. doi: 10.1038/s41541-021-00400-w. NPJ Vaccines. 2021. PMID: 34811393 Free PMC article.
-
Cellular Id1 inhibits hepatitis B virus transcription by interacting with the novel covalently closed circular DNA-binding protein E2F4.Int J Biol Sci. 2022 Jan 1;18(1):65-81. doi: 10.7150/ijbs.62106. eCollection 2022. Int J Biol Sci. 2022. PMID: 34975318 Free PMC article.
-
Heterologous prime-boost immunization with vesiculovirus-based vectors expressing HBV Core antigen induces CD8+ T cell responses in naïve and persistently infected mice and protects from challenge.Antiviral Res. 2019 Aug;168:156-167. doi: 10.1016/j.antiviral.2019.05.014. Epub 2019 May 30. Antiviral Res. 2019. PMID: 31153968 Free PMC article.
-
High antigen burden drives CD8+ T cell dysfunction in a mouse model of chronic hepatitis B virus infection.J Virol. 2025 Jul 22;99(7):e0071125. doi: 10.1128/jvi.00711-25. Epub 2025 Jun 12. J Virol. 2025. PMID: 40503880 Free PMC article.
References
-
- World Health Organization. 2018. Hepatitis B fact sheet. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs204/en/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

