Practical Guidance for Clinical Microbiology Laboratories: Viruses Causing Acute Respiratory Tract Infections
- PMID: 30541871
- PMCID: PMC6302358
- DOI: 10.1128/CMR.00042-18
Practical Guidance for Clinical Microbiology Laboratories: Viruses Causing Acute Respiratory Tract Infections
Abstract
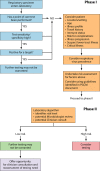
Respiratory viral infections are associated with a wide range of acute syndromes and infectious disease processes in children and adults worldwide. Many viruses are implicated in these infections, and these viruses are spread largely via respiratory means between humans but also occasionally from animals to humans. This article is an American Society for Microbiology (ASM)-sponsored Practical Guidance for Clinical Microbiology (PGCM) document identifying best practices for diagnosis and characterization of viruses that cause acute respiratory infections and replaces the most recent prior version of the ASM-sponsored Cumitech 21 document, Laboratory Diagnosis of Viral Respiratory Disease, published in 1986. The scope of the original document was quite broad, with an emphasis on clinical diagnosis of a wide variety of infectious agents and laboratory focus on antigen detection and viral culture. The new PGCM document is designed to be used by laboratorians in a wide variety of diagnostic and public health microbiology/virology laboratory settings worldwide. The article provides guidance to a rapidly changing field of diagnostics and outlines the epidemiology and clinical impact of acute respiratory viral infections, including preferred methods of specimen collection and current methods for diagnosis and characterization of viral pathogens causing acute respiratory tract infections. Compared to the case in 1986, molecular techniques are now the preferred diagnostic approaches for the detection of acute respiratory viruses, and they allow for automation, high-throughput workflows, and near-patient testing. These changes require quality assurance programs to prevent laboratory contamination as well as strong preanalytical screening approaches to utilize laboratory resources appropriately. Appropriate guidance from laboratorians to stakeholders will allow for appropriate specimen collection, as well as correct test ordering that will quickly identify highly transmissible emerging pathogens.
Keywords: clinical; guidance; laboratory; respiratory; virus.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Greenberg SB, Krilov LR. 1986. Cumitech 21, Laboratory diagnosis of respiratory disease. Coordinating ed., Drew WL, Rubin SJ. American Society for Microbiology, Washington, DC.
-
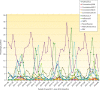
- New Zealand Ministry of Health. 2 October 2016. Community and hospital surveillance: ILI, SARI, influenza and respiratory pathogens. https://surv.esr.cri.nz/PDF_surveillance/Virology/FluWeekRpt/2016/FluWee....
-
- Public Health Agency of Canada. 6 January 2017. FluWatch report. http://healthycanadians.gc.ca/publications/diseases-conditions-maladies-....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

