Extent of MHC Clustering Regulates Selectivity and Effectiveness of T Cell Responses
- PMID: 30541879
- PMCID: PMC6362847
- DOI: 10.4049/jimmunol.1801196
Extent of MHC Clustering Regulates Selectivity and Effectiveness of T Cell Responses
Abstract
MHC proteins that present peptide ligands for recognition by TCR form nanoscale clusters on the cell membrane of APCs. How the extent of MHC clustering controls productive TCR engagement and TCR-mediated signaling has not been systematically studied. To evaluate the role of MHC clustering, we exploited nanoscale discoidal membrane mimetics (nanolipoprotein particles) to capture and present peptide-MHC (pMHC) ligands at various densities. We examined the binding of these model membrane clusters to the surface of live human CD8+ T cells and the subsequent triggering of intracellular signaling. The data demonstrate that the proximity of pMHC ligands, high association rate of CD8-MHC interactions, and relatively long lifetime of cognate TCR-pMHC complexes emerge as essential parameters, explaining the significance of MHC clustering. Rapid rebinding of CD8 to MHC suggests a dual role of CD8 in facilitating the T cells' hunt for a rare foreign pMHC ligand and the induction of rapid T cell response. Thus, our findings provide a new understanding of how MHC clustering influences multivalent interactions of pMHC ligands with CD8 and TCR on live T cells that regulate Ag recognition, kinetics of intracellular signaling, and the selectivity and efficiency of T cell responses.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures
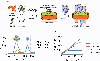


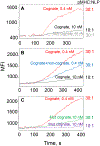
References
-
- Lebedeva T, Anikeeva N, Kalams SA, Walker BD, Gaidarov I, Keen JH, and Sykulev Y. 2004. Major histocompatibility complex class I-intercellular adhesion molecule-1 association on the surface of target cells: implications for antigen presentation to cytotoxic T lymphocytes. Immunology 113: 460–471. - PMC - PubMed
-
- Lebedeva T, Dustin ML, and Sykulev Y. 2005. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol 17: 251–258. - PubMed
-
- Ferez M, Castro M, Alarcon B, and van Santen HM. 2014. Cognate peptide-MHC complexes are expressed as tightly apposed nanoclusters in virus-infected cells to allow TCR crosslinking. J Immunol 192: 52–58. - PubMed
-
- Abbas AK, Dorf ME, Karnovsky MJ, and Unanue ER. 1976. The distribution of Ia antigens on the surfaces of lymphocytes. J Immunol 116: 371–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

