Electrostatics, proton sensor, and networks governing the gating transition in GLIC, a proton-gated pentameric ion channel
- PMID: 30541892
- PMCID: PMC6310827
- DOI: 10.1073/pnas.1813378116
Electrostatics, proton sensor, and networks governing the gating transition in GLIC, a proton-gated pentameric ion channel
Abstract
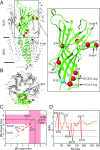
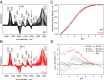
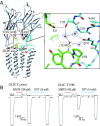
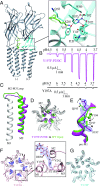
The pentameric ligand-gated ion channel (pLGIC) from Gloeobacter violaceus (GLIC) has provided insightful structure-function views on the permeation process and the allosteric regulation of the pLGICs family. However, GLIC is activated by pH instead of a neurotransmitter and a clear picture for the gating transition driven by protons is still lacking. We used an electrostatics-based (finite difference Poisson-Boltzmann/Debye-Hückel) method to predict the acidities of all aspartic and glutamic residues in GLIC, both in its active and closed-channel states. Those residues with a predicted pKa close to the experimental pH50 were individually replaced by alanine and the resulting variant receptors were titrated by ATR/FTIR spectroscopy. E35, located in front of loop F far away from the orthosteric site, appears as the key proton sensor with a measured individual pKa at 5.8. In the GLIC open conformation, E35 is connected through a water-mediated hydrogen-bond network first to the highly conserved electrostatic triad R192-D122-D32 and then to Y197-Y119-K248, both located at the extracellular domain-transmembrane domain interface. The second triad controls a cluster of hydrophobic side chains from the M2-M3 loop that is remodeled during the gating transition. We solved 12 crystal structures of GLIC mutants, 6 of them being trapped in an agonist-bound but nonconductive conformation. Combined with previous data, this reveals two branches of a continuous network originating from E35 that reach, independently, the middle transmembrane region of two adjacent subunits. We conclude that GLIC's gating proceeds by making use of loop F, already known as an allosteric site in other pLGICs, instead of the classic orthosteric site.
Keywords: allosteric modulation; electrostatic networks; pH activation; pentameric ligand-gated ion channel; proton sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Corringer P-J, et al. Structure and pharmacology of pentameric receptor channels: From bacteria to brain. Structure. 2012;20:941–956. - PubMed
-
- Sauguet L, Shahsavar A, Delarue M. Crystallographic studies of pharmacological sites in pentameric ligand-gated ion channels. Biochim Biophys Acta. 2015;1850:511–523. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

