Neurod1 Is Essential for the Primary Tonotopic Organization and Related Auditory Information Processing in the Midbrain
- PMID: 30541910
- PMCID: PMC6363931
- DOI: 10.1523/JNEUROSCI.2557-18.2018
Neurod1 Is Essential for the Primary Tonotopic Organization and Related Auditory Information Processing in the Midbrain
Abstract
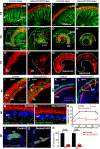
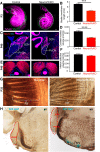
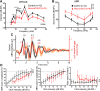
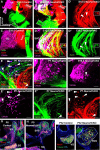
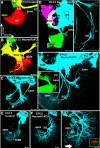
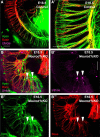
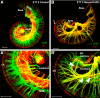
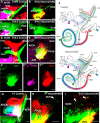
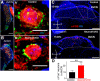
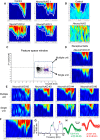
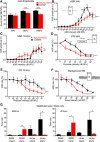
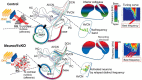
Hearing depends on extracting frequency, intensity, and temporal properties from sound to generate an auditory map for acoustical signal processing. How physiology intersects with molecular specification to fine tune the developing properties of the auditory system that enable these aspects remains unclear. We made a novel conditional deletion model that eliminates the transcription factor NEUROD1 exclusively in the ear. These mice (both sexes) develop a truncated frequency range with no neuroanatomically recognizable mapping of spiral ganglion neurons onto distinct locations in the cochlea nor a cochleotopic map presenting topographically discrete projections to the cochlear nuclei. The disorganized primary cochleotopic map alters tuning properties of the inferior colliculus units, which display abnormal frequency, intensity, and temporal sound coding. At the behavioral level, animals show alterations in the acoustic startle response, consistent with altered neuroanatomical and physiological properties. We demonstrate that absence of the primary afferent topology during embryonic development leads to dysfunctional tonotopy of the auditory system. Such effects have never been investigated in other sensory systems because of the lack of comparable single gene mutation models.SIGNIFICANCE STATEMENT All sensory systems form a topographical map of neuronal projections from peripheral sensory organs to the brain. Neuronal projections in the auditory pathway are cochleotopically organized, providing a tonotopic map of sound frequencies. Primary sensory maps typically arise by molecular cues, requiring physiological refinements. Past work has demonstrated physiologic plasticity in many senses without ever molecularly undoing the specific mapping of an entire primary sensory projection. We genetically manipulated primary auditory neurons to generate a scrambled cochleotopic projection. Eliminating tonotopic representation to auditory nuclei demonstrates the inability of physiological processes to restore a tonotopic presentation of sound in the midbrain. Our data provide the first insights into the limits of physiology-mediated brainstem plasticity during the development of the auditory system.
Keywords: Neurod1 mutation; auditory pathway; cochlear nucleus; inferior colliculus; plasticity; sensory topographical map.
Copyright © 2019 the authors 0270-6474/19/390984-21$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
