Complex Structural PPT1 Variant Associated with Non-syndromic Canine Retinal Degeneration
- PMID: 30541930
- PMCID: PMC6385984
- DOI: 10.1534/g3.118.200859
Complex Structural PPT1 Variant Associated with Non-syndromic Canine Retinal Degeneration
Abstract
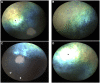
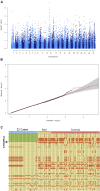
Rod and cone photoreceptors are specialized retinal neurons that have a fundamental role in visual perception, capturing light and transducing it into a neuronal signal. Aberrant functioning of rod and/or cone photoreceptors can ultimately lead to progressive degeneration and eventually blindness. In man, many rod and rod-cone degenerative diseases are classified as forms of retinitis pigmentosa (RP). Dogs also have a comparable disease grouping termed progressive retinal atrophy (PRA). These diseases are generally due to single gene defects and follow Mendelian inheritance.We collected 51 DNA samples from Miniature Schnauzers affected by PRA (average age of diagnosis ∼3.9 ±1 years), as well as from 56 clinically normal controls of the same breed (average age ∼6.6 ±2.8 years). Pedigree analysis suggested monogenic autosomal recessive inheritance of PRA. GWAS and homozygosity mapping defined a critical interval in the first 4,796,806 bp of CFA15. Whole genome sequencing of two affected cases, a carrier and a control identified two candidate variants within the critical interval. One was an intronic SNV in HIVEP3, and the other was a complex structural variant consisting of the duplication of exon 5 of the PPT1 gene along with a conversion and insertion (named PPT1dci ). PPT1dci was confirmed homozygous in a cohort of 22 cases, and 12 more cases were homozygous for the CFA15 haplotype. Additionally, the variant was found homozygous in 6 non-affected dogs of age higher than the average age of onset. The HIVEP3 variant was found heterozygous (n = 4) and homozygous wild-type (n = 1) in cases either homozygous for PPT1dci or for the mapped CFA15 haplotype. We detected the wildtype and three aberrant PPT1 transcripts in isolated white blood cell mRNA extracted from a PRA case homozygous for PPT1dci , and the aberrant transcripts involved inclusion of the duplicated exon 5 and novel exons following the activation of cryptic splice sites. No neurological signs were detected among the dogs homozygous for the PPT1dci variant. Therefore, we propose PPT1dci as causative for a non-syndromic form of PRA (PRA PPT1 ) that shows incomplete penetrance in Miniature Schnauzers, potentially related to the presence of the wild-type transcript. To our knowledge, this is the first case of isolated retinal degeneration associated with a PPT1 variant.
Keywords: PRA; complex variant; dog; palmitoyl protein thioesterase; progressive retinal atrophy; retinal degeneration; whole genome sequencing.
Copyright © 2019 Murgiano et al.
Figures



Comment in
-
Formal commentary.PLoS Genet. 2020 Nov 5;16(11):e1009059. doi: 10.1371/journal.pgen.1009059. eCollection 2020 Nov. PLoS Genet. 2020. PMID: 33151924 Free PMC article. No abstract available.
References
-
- Acland G. M., Ray K., Mellersh C. S., Gu W., Langston A. A., et al. , 1998. Linkage analysis and comparative mapping of canine progressive rod-cone degeneration (prcd) establishes potential locus homology with retinitis pigmentosa (RP17) in humans. Proc. Natl. Acad. Sci. USA 95: 3048–3053. 10.1073/pnas.95.6.3048 - DOI - PMC - PubMed
-
- Aguirre G., Acland G. M., 2006. Models, Mutants and Man: Searching for Unique Phenotypes and Genes in the Dog Model of Inherited Retinal Degeneration, pp. 291–325 in The Dog and Its Genome, edited by Ostrander E. A., Giger U., Lindblad-Toh K. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Aguirre G., O’Brien P., 1986. Morphological and biochemical studies of canine progressive rod-cone degeneration. 3H-fucose autoradiography. Invest. Ophthalmol. Vis. Sci. 27: 635–655. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
