Lysophospholipids in laboratory medicine
- PMID: 30541965
- PMCID: PMC6374142
- DOI: 10.2183/pjab.94.025
Lysophospholipids in laboratory medicine
Abstract
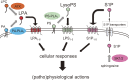
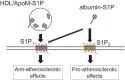
Lysophospholipids (LPLs), such as lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P), and lysophosphatidylserine (LysoPS), are attracting attention as second-generation lipid mediators. In our laboratory, the functional roles of these lipid mediators and the mechanisms by which the levels of these mediators are regulated in vivo have been studied. Based on these studies, the clinical introduction of assays for LPLs and related proteins has been pursued and will be described in this review. Although assays of these lipids themselves are possible, autotaxin (ATX), apolipoprotein M (ApoM), and phosphatidylserine-specific phospholipase A1 (PS-PLA1) are more promising as alternate biomarkers for LPA, S1P, and LysoPS, respectively. Presently, ATX, which produces LPA through its lysophospholipase D activity, has been shown to be a useful laboratory test for the diagnosis and staging of liver fibrosis, whereas PS-PLA1 and ApoM are considered to be promising clinical markers reflecting the in vivo actions induced by LysoPS and S1P.
Keywords: apolipoprotein M; autotaxin; lysophosphatidic acid; lysophosphatidylserine; lysophospholipids; sphingosine 1-phosphate.
Figures


Similar articles
-
[The Laboratory Medicine of Lipid Mediators].Rinsho Byori. 2015 Oct;63(10):1183-6. Rinsho Byori. 2015. PMID: 26897854 Review. Japanese.
-
Lysophospholipids and their producing enzymes: Their pathological roles and potential as pathological biomarkers.Pharmacol Ther. 2023 Jun;246:108415. doi: 10.1016/j.pharmthera.2023.108415. Epub 2023 Apr 13. Pharmacol Ther. 2023. PMID: 37061204 Review.
-
Association between serum autotaxin or phosphatidylserine-specific phospholipase A1 levels and melanoma.J Dermatol. 2018 May;45(5):571-579. doi: 10.1111/1346-8138.14278. Epub 2018 Mar 3. J Dermatol. 2018. PMID: 29500864
-
Current Knowledge on the Biology of Lysophosphatidylserine as an Emerging Bioactive Lipid.Cell Biochem Biophys. 2021 Sep;79(3):497-508. doi: 10.1007/s12013-021-00988-9. Epub 2021 Jun 15. Cell Biochem Biophys. 2021. PMID: 34129148 Free PMC article. Review.
-
Analysis of glycero-lysophospholipids in gastric cancerous ascites.J Lipid Res. 2017 Apr;58(4):763-771. doi: 10.1194/jlr.P072090. Epub 2017 Jan 31. J Lipid Res. 2017. PMID: 28143894 Free PMC article.
Cited by
-
Dynamic modulations of urinary sphingolipid and glycerophospholipid levels in COVID-19 and correlations with COVID-19-associated kidney injuries.J Biomed Sci. 2022 Nov 10;29(1):94. doi: 10.1186/s12929-022-00880-5. J Biomed Sci. 2022. PMID: 36357929 Free PMC article.
-
Urine autotaxin levels reflect the disease activity of sarcoidosis.Sci Rep. 2022 Mar 14;12(1):4372. doi: 10.1038/s41598-022-08388-6. Sci Rep. 2022. PMID: 35288647 Free PMC article.
-
Challenges and Inconsistencies in Using Lysophosphatidic Acid as a Biomarker for Ovarian Cancer.Cancers (Basel). 2019 Apr 11;11(4):520. doi: 10.3390/cancers11040520. Cancers (Basel). 2019. PMID: 30979045 Free PMC article.
-
Determination of Choline-Containing Compounds in Rice Bran Fermented with Aspergillus oryzae Using Liquid Chromatography/Tandem Mass Spectrometry.Mass Spectrom (Tokyo). 2024;13(1):A0151. doi: 10.5702/massspectrometry.A0151. Epub 2024 Aug 8. Mass Spectrom (Tokyo). 2024. PMID: 39161737 Free PMC article.
-
LDL subclass lipidomics in atherogenic dyslipidemia: effect of statin therapy on bioactive lipids and dense LDL.J Lipid Res. 2020 Jun;61(6):911-932. doi: 10.1194/jlr.P119000543. Epub 2020 Apr 15. J Lipid Res. 2020. PMID: 32295829 Free PMC article.
References
-
- Aikawa S., Hashimoto T., Kano K., Aoki J. (2015) Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 157, 81–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

