Rapid detection of metastatic lymph nodes of colorectal cancer with a gamma-glutamyl transpeptidase-activatable fluorescence probe
- PMID: 30542087
- PMCID: PMC6290796
- DOI: 10.1038/s41598-018-36062-3
Rapid detection of metastatic lymph nodes of colorectal cancer with a gamma-glutamyl transpeptidase-activatable fluorescence probe
Abstract
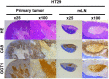
Rapid diagnosis of metastatic lymph nodes (mLNs) of colorectal cancer (CRC) is desirable either intraoperatively or in resected fresh specimens. We have developed a series of activatable fluorescence probes for peptidase activities that are specifically upregulated in various tumors. We aimed to discover a target enzyme for detecting mLNs of CRC. Among our probes, we found that gGlu-HMRG, a gamma-glutamyl transpeptidase (GGT)-activatable fluorescence probe, could detect mLNs. This was unexpected, because we have previously reported that gGlu-HMRG could not detect primary CRC. We confirmed that the GGT activity of mLNs was high, whereas that of non-metastatic lymph nodes and CRC cell lines was low. We investigated the reason why GGT activity was upregulated in mLNs, and found that GGT was induced under conditions of hypoxia or low nutritional status. We utilized this feature to achieve rapid detection of mLNs with gGlu-HMRG. GGT appears to be a promising candidate enzyme for fluorescence imaging of mLNs.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kantorová I, et al. Routine 18F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–1788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

