Baculovirus as an efficient vector for gene delivery into mosquitoes
- PMID: 30542209
- PMCID: PMC6290771
- DOI: 10.1038/s41598-018-35463-8
Baculovirus as an efficient vector for gene delivery into mosquitoes
Abstract
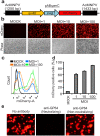
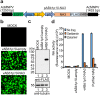
Efficient gene delivery technologies play an essential role in the gene functional analyses that are necessary for basic and applied researches. Mosquitoes are ubiquitous insects, responsible for transmitting many deadly arboviruses causing millions of human deaths every year. The lack of efficient and flexible gene delivery strategies in mosquitoes are among the major hurdles for the study of mosquito biology and mosquito-pathogen interactions. We found that Autographa californica multiple nucleopolyhedrovirus (AcMNPV), the type baculovirus species, can efficiently transduce mosquito cells without viral propagation, allowing high level gene expression upon inducement by suitable promoters without obvious negative effects on cell propagation and viability. AcMNPV transduces into several mosquito cell types, efficiently than in commonly used mammalian cell lines and classical plasmid DNA transfection approaches. We demonstrated the application of this system by expressing influenza virus neuraminidase (NA) into mosquito hosts. Moreover, AcMNPV can transduce both larvae and adults of essentially all blood-sucking mosquito genera, resulting in bright fluorescence in insect bodies with little or no tissue barriers. Our experiments establish baculovirus as a convenient and powerful gene delivery vector in vitro and in vivo that will greatly benefit research into mosquito gene regulation, development and the study of mosquito-borne viruses.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Major Hurdle for Effective Baculovirus Transduction into Mammalian Cells Is Passing Early Endosomes.J Virol. 2019 Jul 17;93(15):e00709-19. doi: 10.1128/JVI.00709-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092570 Free PMC article.
-
Evaluation of the Nucleopolyhedrovirus of Anticarsia gemmatalis as a Vector for Gene Therapy in Mammals.Curr Gene Ther. 2021;21(2):177-189. doi: 10.2174/1566523220999201217155945. Curr Gene Ther. 2021. PMID: 33334288
-
Cell-dependent production of polyhedra and virion occlusion of Autographa californica multiple nucleopolyhedrovirus fp25k mutants in vitro and in vivo.J Gen Virol. 2013 Jan;94(Pt 1):177-186. doi: 10.1099/vir.0.045591-0. Epub 2012 Sep 19. J Gen Virol. 2013. PMID: 22993192
-
[A novel application of baculovirus in mammalian gene therapy].Wei Sheng Wu Xue Bao. 2006 Aug;46(4):668-72. Wei Sheng Wu Xue Bao. 2006. PMID: 17037077 Review. Chinese.
-
Expanding the canon: Non-classical mosquito genes at the interface of arboviral infection.Insect Biochem Mol Biol. 2019 Jun;109:72-80. doi: 10.1016/j.ibmb.2019.04.004. Epub 2019 Apr 7. Insect Biochem Mol Biol. 2019. PMID: 30970277 Review.
Cited by
-
Facile method for delivering chikungunya viral replicons into mosquitoes and mammalian cells.Sci Rep. 2021 Jun 10;11(1):12321. doi: 10.1038/s41598-021-91830-y. Sci Rep. 2021. PMID: 34112897 Free PMC article.
-
A novel method for generating baculovirus bacmids using EGFP-mediated purification and linearization.Biotechnol Lett. 2025 Jul 17;47(4):77. doi: 10.1007/s10529-025-03619-y. Biotechnol Lett. 2025. PMID: 40670866
-
Antigenicity and immunogenicity of chikungunya virus-like particles from mosquito cells.Appl Microbiol Biotechnol. 2023 Jan;107(1):219-232. doi: 10.1007/s00253-022-12280-8. Epub 2022 Nov 25. Appl Microbiol Biotechnol. 2023. PMID: 36434113 Free PMC article.
-
The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review.Vaccines (Basel). 2025 May 23;13(6):556. doi: 10.3390/vaccines13060556. Vaccines (Basel). 2025. PMID: 40573887 Free PMC article. Review.
-
Identifying the Gut Virome of Diaphorina citri from Florida Groves.Insects. 2023 Feb 8;14(2):166. doi: 10.3390/insects14020166. Insects. 2023. PMID: 36835735 Free PMC article.
References
-
- WHO. Malaria Fact Sheet. World Health Organization (WHO) (2017).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- MOST 106-2321-B-001-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-2321-B-001-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-2321-B-001-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-2321-B-001-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-2321-B-001-012/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST 106-3114-B-033-001/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- MOST105-2321-B-001-040/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)/International
- NHRI-106A1-MRCO-0217171/National Health Research Institutes (NHRI)/International
- 022361/Academia Sinica/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

