Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated With Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice
- PMID: 30542282
- PMCID: PMC6277937
- DOI: 10.3389/fphar.2018.01332
Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated With Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice
Abstract
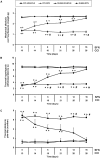
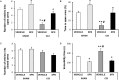
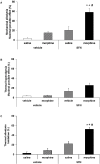
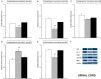
Chronic neuropathic pain is associated with anxiety- and depressive-like disorders. Its treatment remains a serious clinical problem due to the lack of efficacy of the available therapeutic modalities. We investigated if the activation of the transcription factor Nrf2 could modulate the nociceptive and emotional disorders associated with persistent neuropathic pain and potentiated the analgesic activity of morphine. The possible mechanisms implicated in these effects have been also evaluated. Therefore, in C57BL/6 mice with neuropathic pain induced by the chronic constriction of the sciatic nerve (CCI), we assessed the antinociceptive, anxiolytic, and anti-depressant effects of the repeated intraperitoneal administration of a Nrf2 inducer, sulforaphane (SFN), and the effects of this treatment on the local antinociceptive actions of morphine. The protein levels of Nrf2, heme oxygenase 1 (HO-1), NAD(P)H:quinone oxidoreductase-1 (NQO1), CD11b/c (a microglial activator marker), mitogen-activated protein kinases (MAPK) and μ opioid receptors (MOR) in the spinal cord, prefrontal cortex and hippocampus from mice, at 28 days after CCI, were also evaluated. Our results showed that the repeated administration of SFN besides inhibiting nociceptive responses induced by sciatic nerve injury also diminished the anxiety- and depressive-like behaviors associated with persistent neuropathic pain. Moreover, SFN treatment normalized oxidative stress by inducing Nrf2/HO-1 signaling, reduced microglial activation and JNK, ERK1/2, p-38 phosphorylation induced by sciatic nerve injury in the spinal cord and/or hippocampus and prefrontal cortex. Interestingly, treatment with SFN also potentiated the antiallodynic effects of morphine in sciatic nerve-injured mice by regularizing the down regulation of MOR in the spinal cord and/or hippocampus. This study suggested that treatment with SFN might be an interesting approach for the management of persistent neuropathic pain and comorbidities associated as well as to improve the analgesic actions of morphine.
Keywords: Nrf2 transcription factor; analgesia; anxiety; chronic pain; depression; opioids; oxidative stress.
Figures
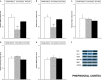
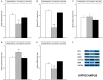

References
-
- Alba-Delgado C., Cebada-Aleu A., Mico J. A., Berrocoso E. (2016). Comorbid anxiety-like behavior and locus coeruleus impairment in diabetic peripheral neuropathy: a comparative study with the chronic constriction injury model. Prog. Neuropsychopharmacol. Biol. Psychiatry 71 45–56. 10.1016/j.pnpbp.2016.06.007 - DOI - PubMed
-
- Bennett G. J., Xie Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33 87–107. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

