Electroacupuncture stimulates the proliferation and differentiation of endogenous neural stem cells in a rat model of ischemic stroke
- PMID: 30542450
- PMCID: PMC6257304
- DOI: 10.3892/etm.2018.6848
Electroacupuncture stimulates the proliferation and differentiation of endogenous neural stem cells in a rat model of ischemic stroke
Abstract
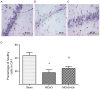
Electroacupuncture (EA) may stimulate neurogenesis in animal models of ischemic stroke; however, the associated mechanisms are not clear. The present study aimed to evaluate the neurogenesis efficacy of EA on ischemic stroke and the underlying associated mechanisms. A model of middle cerebral artery occlusion (MCAO) was employed as the rat model of brain ischemia and reperfusion. EA treatment at the GV20 (Baihui) and GV14 (Dazhui) acupoints was conducted for 30 min daily following MCAO. Immunofluorescence was performed to measure the number of bromodeoxyuridine (BrdU)/nestin- or BrdU/doublecortin (DCX)-positive cells in the sham, MCAO and MCAO + EA groups. Results indicated that EA stimulation significantly decreased the neurological score and neuronal loss in rats in the MCAO group (both P<0.05). Furthermore, immunostaining assays indicated that BrdU/nestin- and BrdU/DCX-positive cells in EA-treated rats were significantly increased (P<0.05) when compared with the rats in the MCAO group, indicating EA may induce the proliferation and differentiation of endogenous neural stem cells (eNSCs) during cerebral ischemia-reperfusion. In addition, EA treatment significantly enhanced the protein expression levels of plasticity-related gene 5 (PRG5), a critical neurogenesis factor, and significantly decreased the protein expression levels of three neurogenesis inhibiting molecules, NogoA, lysophosphatidic acid and RhoA (all P<0.05). These results suggested that EA promotes the proliferation and differentiation of eNSCs, likely through modulating PRG5/RhoA signaling.
Keywords: RhoA; cerebral ischemia; electroacupuncture; neural stem cells; plasticity-related gene 5.
Figures




Similar articles
-
The roles, mechanism, and mobilization strategy of endogenous neural stem cells in brain injury.Front Aging Neurosci. 2022 Aug 17;14:924262. doi: 10.3389/fnagi.2022.924262. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062152 Free PMC article. Review.
-
[Effect of Electroacupuncture on the Expression of Hippocampal eNSCs in MCAO Model Rats].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2017 Feb;37(2):198-203. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2017. PMID: 30650273 Chinese.
-
Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia.PLoS One. 2014 Feb 24;9(2):e90000. doi: 10.1371/journal.pone.0090000. eCollection 2014. PLoS One. 2014. PMID: 24587178 Free PMC article.
-
Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity.PLoS One. 2014 Mar 13;9(3):e91426. doi: 10.1371/journal.pone.0091426. eCollection 2014. PLoS One. 2014. PMID: 24626220 Free PMC article.
-
Bioactive components of Chinese herbal medicine enhance endogenous neurogenesis in animal models of ischemic stroke: A systematic analysis.Medicine (Baltimore). 2016 Oct;95(40):e4904. doi: 10.1097/MD.0000000000004904. Medicine (Baltimore). 2016. PMID: 27749547 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell Application and Its Therapeutic Mechanisms in Intracerebral Hemorrhage.Front Cell Neurosci. 2022 Jun 13;16:898497. doi: 10.3389/fncel.2022.898497. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35769327 Free PMC article. Review.
-
The reporting quality of acupuncture for neurogenesis in experimental ischemic stroke study.Ann Transl Med. 2019 Mar;7(6):123. doi: 10.21037/atm.2019.02.16. Ann Transl Med. 2019. PMID: 31032278 Free PMC article.
-
Electroacupuncture for the treatment of ischemic stroke: A preclinical meta-analysis and systematic review.Neural Regen Res. 2026 Mar 1;21(3):1191-1210. doi: 10.4103/NRR.NRR-D-24-01030. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885673 Free PMC article.
-
The roles, mechanism, and mobilization strategy of endogenous neural stem cells in brain injury.Front Aging Neurosci. 2022 Aug 17;14:924262. doi: 10.3389/fnagi.2022.924262. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062152 Free PMC article. Review.
-
Repetitive transcranial magnetic stimulation increases neurological function and endogenous neural stem cell migration via the SDF-1α/CXCR4 axis after cerebral infarction in rats.Exp Ther Med. 2021 Sep;22(3):1037. doi: 10.3892/etm.2021.10469. Epub 2021 Jul 19. Exp Ther Med. 2021. PMID: 34373723 Free PMC article.
References
-
- Liu D, Wang Z, Zhan J, Zhang Q, Wang J, Zhang Q, Xian X, Luan Q, Hao A. Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells andprotects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacol Biochem Behav. 2014;116:55–63. doi: 10.1016/j.pbb.2013.11.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
