Dendritic cell vaccine with Ag85A enhances anti-colorectal carcinoma immunity
- PMID: 30542467
- PMCID: PMC6257656
- DOI: 10.3892/etm.2018.6851
Dendritic cell vaccine with Ag85A enhances anti-colorectal carcinoma immunity
Abstract
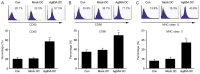
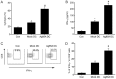
Dendritic cells (DCs) are able to trigger T-cell activation and thus have been considered important for vaccine production against cancers. Vaccines containing DCs have been reported to be effective for developing immunity against cancer cells. The interactions between DCs and auxiliary agents are critical in the development of second-generation vaccines. In the present study, it was evaluated whether Ag85A-mixed DCs could enhance anti-tumor immunity in laboratory mice with colorectal carcinoma. Functional and phenotypic analyses of the effects of Ag85A-mixed DCs were conducted via flow cytometry and measurement of T-cell proliferation. In addition, interferon (IFN)-γ production was assessed. The therapeutic efficacy of DC vaccination for colorectal carcinoma treatment in mice was investigated. It was identified that Ag85A-mixed DCs exhibited strong upregulation of CD80, CD86 and major histocompatibility complex class II. Cytotoxic T-lymphocytes with CT26-primed Ag85A-DCs were indicated to induce stronger responses against CT26 tumor cells and trigger IFN-γ production. Furthermore, the Ag85A-mixed DC vaccine exerted a considerable inhibitory effect on tumor progression in mice as compared with the control group. Therefore, DCs in combination with the Ag85A gene may reinforce anti-colorectal carcinoma immunity. The current study provides a novel potential strategy for cancer treatment by enhancing immunity via Ag85A-mixed DC vaccination.
Keywords: antigen 85A; colorectal carcinoma; dendritic cells; major histocompatibility complex class II.
Figures


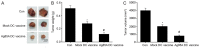
References
-
- Bogach J, Levine O, Parpia S, Valencia M, Ruo L, Serrano P. Does the addition of biologic agents to chemotherapy in patients with unresectable colorectal cancer metastases result in a higher proportion of patients undergoing resection? A systematic review and meta-analysis. J Gastrointest Surg. 2018;22:523–528. doi: 10.1007/s11605-017-3640-6. - DOI - PubMed
-
- Kwakman JJM, Vink G, Vestjens JH, Beerepoot LV, de Groot JW, Jansen RL, Opdam FL, Boot H, Creemers GJ, van Rooijen JM, et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: Real-life data from The Netherlands. Int J ClinOncol. 2018;23:482–489. - PMC - PubMed
-
- Mohammadian M, Zeynali S, Azarbaijani AF, Khadem AM, Kheradmand F. Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines. Res Pharm Sci1. 2017;12:517–525. doi: 10.4103/1735-5362.217432. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
