Atorvastatin reverses the dysfunction of human umbilical vein endothelial cells induced by angiotensin II
- PMID: 30542486
- PMCID: PMC6257519
- DOI: 10.3892/etm.2018.6846
Atorvastatin reverses the dysfunction of human umbilical vein endothelial cells induced by angiotensin II
Abstract
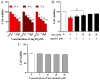
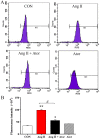
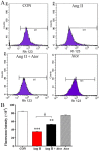
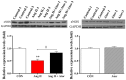
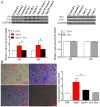
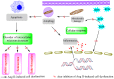
Statins exert pleiotropic effects on endothelial cells, in addition to lowering cholesterol. This study evaluated angiotensin II (Ang II)-induced dysfunction in human umbilical vein endothelial cells (HUVECs), and the effects of atorvastatin (Ator) on induced HUVECs in vitro. The cytotoxicity of Ang II and Ator was determined by the MTT assay. A series of cellular responses were screened, including oxidative stress, cellular apoptosis, inflammatory response, autophagy, expression of endothelial nitric oxide synthase and the angiogenic function of HUVECs. Ator returned these cellular responses to a normal level. The present study also examined cellular organelle dysfunction. In HUVECs, Ang II triggered mitochondrial damage, as demonstrated by a decreased mitochondrial membrane potential, while Ator attenuated this Ang II-induced damage. The observed cellular dysfunction may cause endothelial senescence due to excessive cell injury. The current study examined several aging markers, which revealed that these disorders of cellular functions triggered endothelial senescence, which was delayed by Ator. Ator also suppressed Ang II-induced angiogenesis damage. The data presented in this study strongly suggested that Ang II induced a series of processes that lead to cellular dysfunction in HUVECs, including oxidative stress, inflammation, and mitochondrial damage, leading to apoptosis and endothelial senescence. However, Ator significantly reversed these effects and modulated intracellular stability. The present study indicated that Ator serves an antagonistic role against HUVEC dysfunction and may potentially prevent several diseases, including coronary disease and atherosclerosis, by maintaining cellular homeostasis.
Keywords: atorvastatin; cellular dysfunction; homeostasis; human umbilical vein endothelial cells.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
