Targeting Brd4 for cancer therapy: inhibitors and degraders
- PMID: 30542529
- PMCID: PMC6238758
- DOI: 10.1039/c8md00198g
Targeting Brd4 for cancer therapy: inhibitors and degraders
Abstract
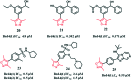
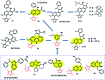
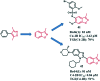
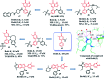
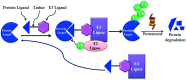
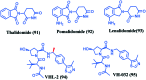
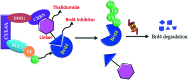
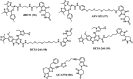
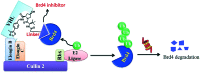
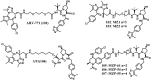
Bromodomain-containing protein 4 (Brd4) plays an important role in mediating the expression of genes involved in cancers and non-cancer diseases such as inflammatory diseases and acute heart failure. Inactivating Brd4 or downregulating its expression inhibits cancer development, leading to the current interest in Brd4 as a promising anticancer drug target. Numerous Brd4 inhibitors have been studied in recent years and some of them are currently in various phases of clinical trials. Recently, selective degradation of target proteins by small bifunctional molecules (PROTACs) has emerged as an attractive drug discovery approach owing to the advantages it could offer over traditional small-molecule inhibitors. A number of Brd4 degraders have been reported and showed more efficient anticancer activities than just protein inhibition. In this review, we will discuss recent findings in the discovery and development of small-molecule inhibitors and degraders that target Brd4 as a potential anticancer agent.
Figures
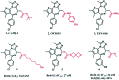
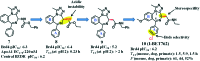
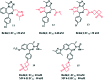
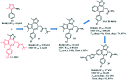
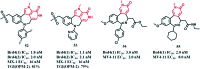
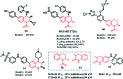
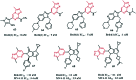
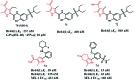
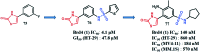
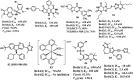
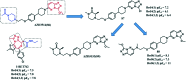
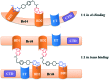

References
-
- Ronen Marmorstein S. Y. R. Curr. Opin. Genet. Dev. 2001;11:155–161. - PubMed
-
- Jenuwein T., Allis C. D. Science. 2001;293:1074–1080. - PubMed
-
- Jaiprakash N. S. S., Sangshett N., Dehghan M. H. G., Shinde D. B. Mini-Rev. Med. Chem. 2013;13:1005–1026. - PubMed
-
- Zeng L., Zhou M. M. FEBS Lett. 2002:124–128. - PubMed
-
- Ferri E., Petosa C., McKenna C. E. Biochem. Pharmacol. 2016;106:1–18. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

