Benzophenone: a ubiquitous scaffold in medicinal chemistry
- PMID: 30542530
- PMCID: PMC6238883
- DOI: 10.1039/c8md00300a
Benzophenone: a ubiquitous scaffold in medicinal chemistry
Abstract
The benzophenone scaffold represents a ubiquitous structure in medicinal chemistry because it is found in several naturally occurring molecules which exhibit a variety of biological activities, such as anticancer, anti-inflammatory, antimicrobial, and antiviral. In addition, various synthetic benzophenone motifs are present in marketed drugs. They also represent important ingredients in perfumes and can act as photoinitiators. This review will provide an overview of benzophenone moieties with medicinal aspects synthesized in the last 15 years and will cover the most potent molecule in each report. In this review, only benzophenones with substitutions on their aryl rings, i.e. diphenyl ketone analogues, have been covered.
Figures























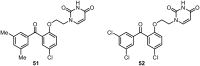
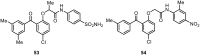




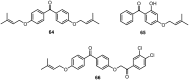







Similar articles
-
Structural diversity and bioactivities of natural benzophenones.Nat Prod Rep. 2014 Sep;31(9):1158-74. doi: 10.1039/c4np00027g. Nat Prod Rep. 2014. PMID: 24972079 Review.
-
C-F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres.Nat Commun. 2024 Jan 10;15(1):419. doi: 10.1038/s41467-023-44653-6. Nat Commun. 2024. PMID: 38199996 Free PMC article.
-
Conformations of substituted benzophenones.Acta Crystallogr B. 2008 Apr;64(Pt 2):206-16. doi: 10.1107/S0108768108000232. Epub 2008 Mar 14. Acta Crystallogr B. 2008. PMID: 18369292
-
Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011-2016).Expert Opin Ther Pat. 2018 Apr;28(4):281-297. doi: 10.1080/13543776.2018.1432596. Epub 2018 Feb 5. Expert Opin Ther Pat. 2018. PMID: 29368977 Review.
-
Synthesis and anti-inflammatory activity of benzophenone analogues.Bioorg Chem. 2004 Aug;32(4):211-22. doi: 10.1016/j.bioorg.2004.04.003. Bioorg Chem. 2004. PMID: 15210336
Cited by
-
More Than Resveratrol: New Insights into Stilbene-Based Compounds.Biomolecules. 2020 Jul 27;10(8):1111. doi: 10.3390/biom10081111. Biomolecules. 2020. PMID: 32726968 Free PMC article. Review.
-
Novel Azo dyes containing a hydrazide-hydrazone moiety for dyeing polyester fabric.Sci Rep. 2025 Feb 5;15(1):4360. doi: 10.1038/s41598-024-83565-3. Sci Rep. 2025. PMID: 39910090 Free PMC article.
-
Garcixanthone E and Garcimangophenone C: New Metabolites from Garcinia mangostana and Their Cytotoxic and Alpha Amylase Inhibitory Potential.Life (Basel). 2022 Nov 14;12(11):1875. doi: 10.3390/life12111875. Life (Basel). 2022. PMID: 36431010 Free PMC article.
-
SAR study of piperidine derivatives as inhibitors of 1,4-dihydroxy-2-naphthoate isoprenyltransferase (MenA) from Mycobacterium tuberculosis.Eur J Med Chem. 2023 Mar 5;249:115125. doi: 10.1016/j.ejmech.2023.115125. Epub 2023 Jan 18. Eur J Med Chem. 2023. PMID: 36682292 Free PMC article.
-
Recent Advances in Chemical Biology Using Benzophenones and Diazirines as Radical Precursors.Molecules. 2020 May 13;25(10):2285. doi: 10.3390/molecules25102285. Molecules. 2020. PMID: 32414020 Free PMC article. Review.
References
-
- Wu S. B., Long C., Kennelly E. J. Nat. Prod. Rep. 2014;31:1158–1174. - PubMed
-
- Acuna U. M., Jancovski N., Kennelly E. J. Curr. Top. Med. Chem. 2009;9:1560–1580. - PubMed
-
- Zhang C., Ondeyka J. G., Herath K. B., Guan Z., Collado J., Platas G., Pelaez F., Leavitt P. S., Gurnett A., Nare B., Liberator P., Singh S. B. J. Nat. Prod. 2005;68:611–613. - PubMed
-
- Bernardi A. P., Ferraz A. B., Albring D. V., Bordignon S. A., Schripsema J., Bridi R., Dutra-Filho C. S., Henriques A. T., von Poser G. L. J. Nat. Prod. 2005;68:784–786. - PubMed
-
- Casu L., Solinas M. N., Saba A. R., Cottiglia F., Caboni P., Floris C., Laconi S., Pompei R., Leonti M. Phytochem. Lett. 2010;3:226–229.
Publication types
LinkOut - more resources
Full Text Sources
Chemical Information

